32008
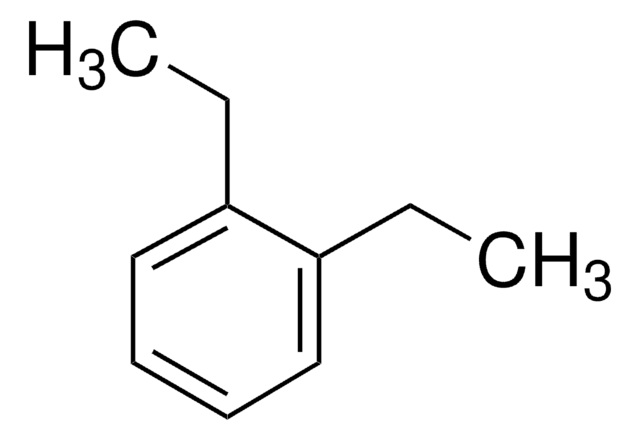
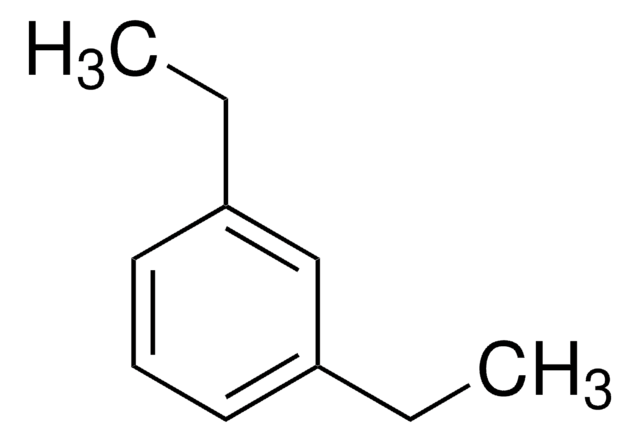
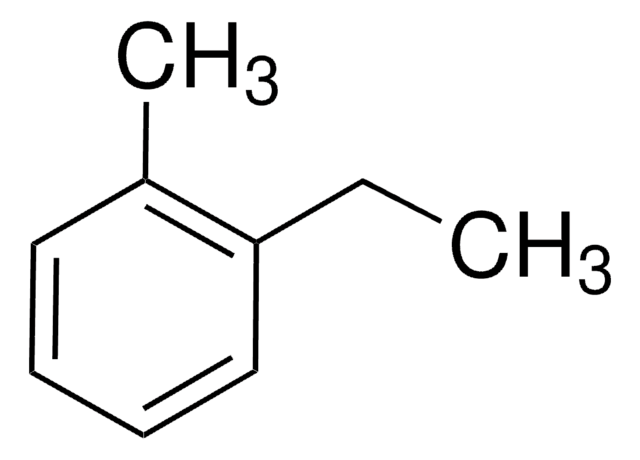
1,2-Diethylbenzene
≥99.0% (GC)
Synonym(s):
o-Diethylbenzene
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
C6H4(C2H5)2
CAS Number:
Molecular Weight:
134.22
Beilstein/REAXYS Number:
1904392
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
≥99.0% (GC)
autoignition temp.
743 °F
refractive index
n20/D 1.502 (lit.)
n20/D 1.503
bp
183 °C (lit.)
mp
−31 °C (lit.)
density
0.88 g/mL at 25 °C (lit.)
SMILES string
CCc1ccccc1CC
InChI
1S/C10H14/c1-3-9-7-5-6-8-10(9)4-2/h5-8H,3-4H2,1-2H3
InChI key
KVNYFPKFSJIPBJ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
1,2-Diethylbenzene can be used as a reactant in the preparation of pseudocyclic diaryliodonium triflates, 5,6-disubstituted indanone intermediates via intramolecular Friedel-Crafts alkylation, dibromide intermediates via radical bromination, and in the dehydrogenative C-H/C-H arylation of indolines.
Application
- Attraction of adult Harmonia axyridis to volatiles of the insectary plant Cnidium monnieri: This study investigates the attraction of the beetle Harmonia axyridis to 1,2-diethylbenzene, indicating potential uses in biological control strategies (Zhiping et al., 2020).
signalword
Warning
hcodes
pcodes
Hazard Classifications
Flam. Liq. 3
Storage Class
3 - Flammable liquids
wgk_germany
WGK 2
flash_point_f
131.0 °F - closed cup
flash_point_c
55 °C - closed cup
ppe
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Formation of 4, 5, 6, 7-tetrahydroisoindoles by palladium-catalyzed hydride reduction
Hou D, et al.
The Journal of Organic Chemistry, 72 (2007)
Exceptionally Mild Palladium (II)-Catalyzed Dehydrogenative C--H/C--H Arylation of Indolines at the C-7 Position under Air
Jiao L, et al.
Organic Letters, 16 (2014)
Synthesis of arylbenziodoxoles using pseudocyclic benziodoxole triflate and arenes
Yoshimura A, et al.
ARKIVOC (Gainesville, FL, United States), 2020 (2021)
Jean-Paul Payan et al.
Archives of toxicology, 82(9), 591-600 (2008-02-07)
The bio-distribution of the neurotoxic 1,2-diethylbenzene (1,2-DEB) was studied in male Sprague-Dawley rats after intravenous administration of [(14)C] 1,2-DEB (1 mg kg(-1)). The highest concentrations of [(14)C] non-volatile metabolites, determined by whole-body auto-radiography, were in the nasal cavity, ethmoid turbinates
I Linhart et al.
Xenobiotica; the fate of foreign compounds in biological systems, 26(12), 1263-1272 (1996-12-01)
1. Biotransformation of 1,2-diethenylbenzene (1) in rat was studied. Five urinary metabolites were isolated by extraction of acid hydrolysed urine and identified by nmr and mass spectroscopy, namely, 1-(2-ethenylphenyl)ethane-1,2-diol (2) 2-ethenylmandelic acid (3), 2-ethenylphenylglyoxylic acid (4), 2-ethenylphenylacetylglycine (5) N-acetyl-S-[1-(2-ethenylphenyl)-2-hydroxyethyl]cysteine (6)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service