325384
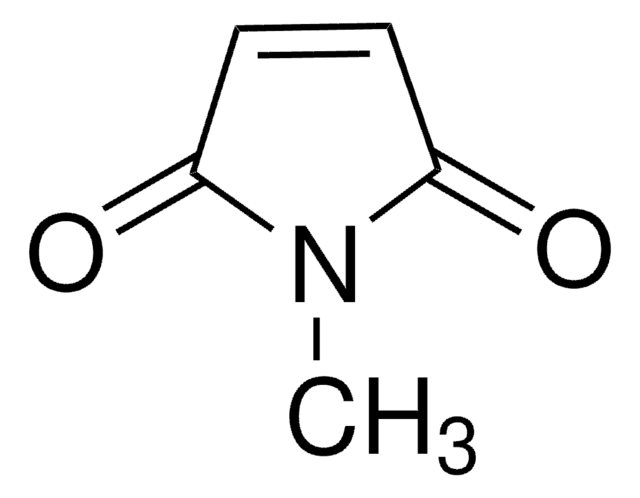
N-Methylsuccinimide
99%
Synonym(s):
1-Methyl-2,5-pyrrolidinedione, N-Methyl-2,5-pyrrolidinedione
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C5H7NO2
CAS Number:
Molecular Weight:
113.11
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
99%
form
solid
bp
234-235 °C (lit.)
mp
61-70 °C (lit.)
functional group
imide
SMILES string
CN1C(=O)CCC1=O
InChI
1S/C5H7NO2/c1-6-4(7)2-3-5(6)8/h2-3H2,1H3
InChI key
KYEACNNYFNZCST-UHFFFAOYSA-N
General description
N-Methylsuccinimide is a metabolite of N-methyl-2-pyrrolidone (NMP) and its presence in in plasma and urine can be used as a biomarker of exposure to NMP. Hydrogen bonded complexes between N-methylsuccinimide and phenols (pKa = 10.2 → 6) are investigated by infrared spectrometry.
Application
N-Methylsuccinimide was employed as model compound to investigate the mechanism of enolization step by density-functional theory (DFT) calculations.
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 2
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Ohgi Takahashi et al.
Chemistry & biodiversity, 7(6), 1349-1356 (2010-06-22)
Racemization of aspartic acid residues in peptides and proteins is assumed to proceed via succinimide intermediates. An enolization of the succinimide intermediate is required for the racemization to occur. In this study, we modeled the enolization step by density-functional theory
N-Methylsuccinimide in plasma and urine as a biomarker of exposure to N-methyl-2-pyrrolidone.
Jonsson BAG and ?kesson B.
International Archives of Occupational and Environmental Health, 74(4), 289-294 (2004)
nfrared Study of the Hydrogen Bond Complexes Between N-Methylsuccinimide and Phenols.
Bruyneel K, et al.
Spectroscopy Letters, 29(4), 739-747 (1996)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service