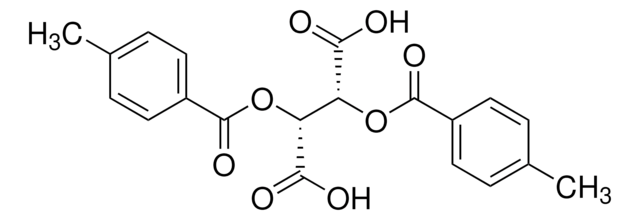
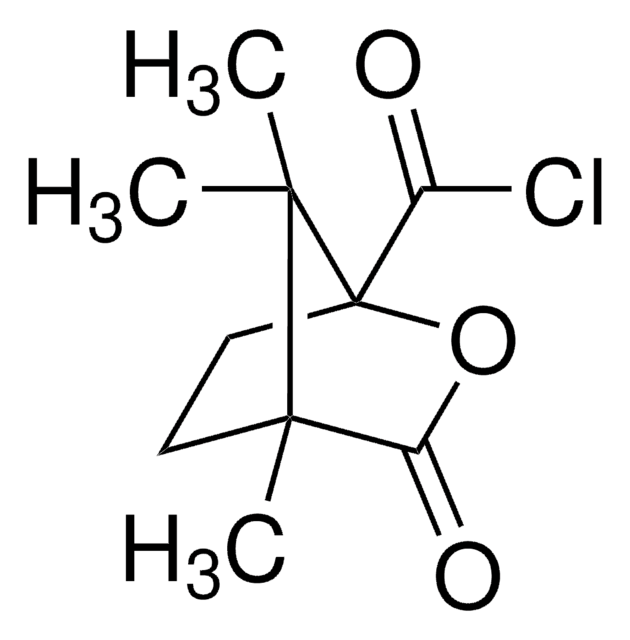
358924
(+)-O,O′-Diacetyl-L-tartaric anhydride
97%
Synonym(s):
(+)-Diacetyl-L-tartaric anhydride
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C8H8O7
CAS Number:
Molecular Weight:
216.14
Beilstein/REAXYS Number:
87315
EC Number:
MDL number:
UNSPSC Code:
12352005
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
97%
optical activity
[α]20/D +59°, c = 6 in acetone
mp
130-135 °C (lit.)
functional group
anhydride
ester
storage temp.
2-8°C
SMILES string
CC(=O)O[C@@H]1[C@@H](OC(C)=O)C(=O)OC1=O
InChI
1S/C8H8O7/c1-3(9)13-5-6(14-4(2)10)8(12)15-7(5)11/h5-6H,1-2H3/t5-,6-/m1/s1
InChI key
XAKITKDHDMPGPW-PHDIDXHHSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
(+)-O,O′-Diacetyl-L-tartaric anhydride is an HPLC derivatization reagent for UV/Vis detection. It is mainly employed as a reagent for the chiral derivatization of amino alcohols. It also reacts with alkanoamines in aprotic medium containing trichloroacetic acid and produces tartaric acid monoesters.
Application
(+)-O,O′-Diacetyl-L-tartaric anhydride may be used as a chiral derivatizating agent in the following:
- determination of enantiomeric vigabatrin in mouse serum samples using ultra-high performance liquid chromatography-quadrupole-time-of-flight mass spectrometry (UHPLC-Q-TOF-M)
- determination of trantinterol in rat plasma by ultra performance liquid chromatography–electrospray ionization mass spectrometry (UPLC–MS/MS)
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Determination of enantiomeric vigabatrin by derivatization with diacetyl-l-tartaric anhydride followed by ultra-high performance liquid chromatography-quadrupole-time-of-flight mass spectrometry
Zhao J, et al.
Journal of Chromatography. B, Biomedical Sciences and Applications, 1040, 199-207 (2017)
William M Oldham et al.
Bio-protocol, 6(16) (2017-06-03)
Two enantiomers of 2-hydroxyglutarate (2HG), L (L2HG) and D (D2HG), are metabolites of unknown function in mammalian cells that were initially associated with separate and rare inborn errors of metabolism resulting in increased urinary excretion of 2HG linked to neurological
W Lindner et al.
Journal of chromatography, 487(2), 375-383 (1989-02-24)
A sensitive high-performance liquid chromatographic method was developed for the stereoselective assay of (R)- and (S)-propranolol in human plasma. The method involves diethyl ether extraction of the drugs and a racemic internal standard, N-tert.-butylpropranolol, followed by derivatization of the compounds
D R Brocks et al.
Journal of pharmaceutical and biomedical analysis, 13(7), 911-918 (1995-06-01)
A stereospecific liquid chromatographic (LC) assay was developed for the quantification of the antimalarial drug, halofantrine, in human plasma. Following protein precipitation with acetonitrile, the enantiomers of halofantrine were extracted from human plasma using ammonium hydroxide and tert-butyl methyl ether-hexane.
Enantioselective determination of trantinterol in rat plasma by ultra performance liquid chromatography?electrospray ionization mass spectrometry after derivatization
Yang J, et al.
Talanta, 79(5), 1204-1208 (2009)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service