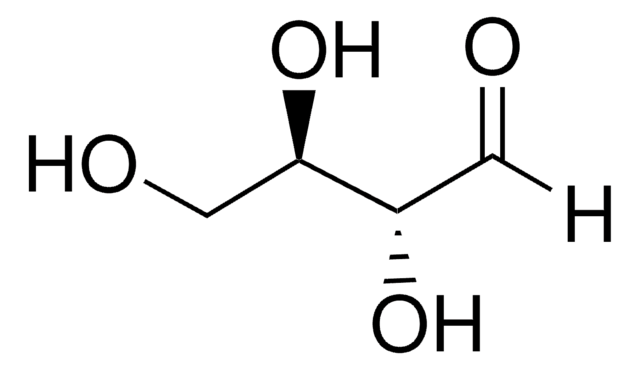
377619
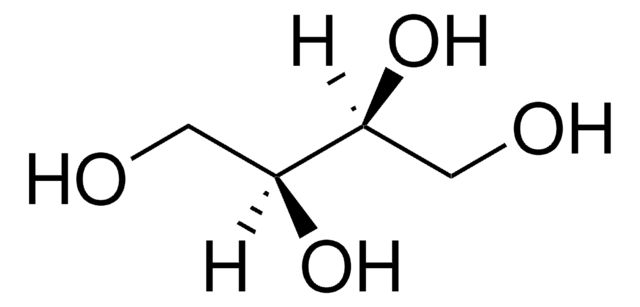
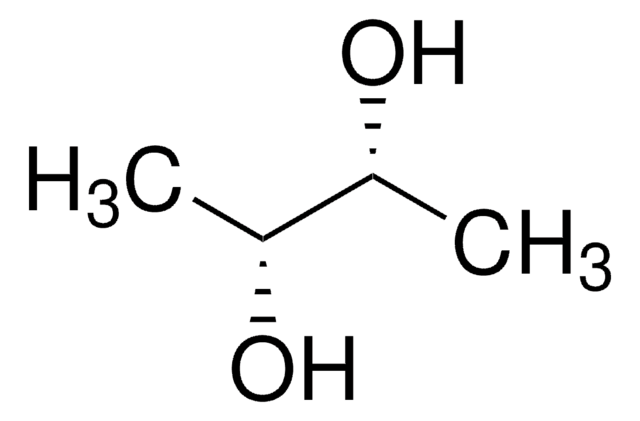
D-Threitol
99%
Synonym(s):
(2R,3R)-1,2,3,4-Butanetetrol
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
HOCH2[CH(OH)]2CH2OH
CAS Number:
Molecular Weight:
122.12
Beilstein/REAXYS Number:
1719752
MDL number:
UNSPSC Code:
12352201
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
99%
form
solid
optical activity
[α]20/D −14°, c = 2 in ethanol
mp
88-90 °C (lit.)
SMILES string
OC[C@@H](O)[C@H](O)CO
InChI
1S/C4H10O4/c5-1-3(7)4(8)2-6/h3-8H,1-2H2/t3-,4-/m1/s1
InChI key
UNXHWFMMPAWVPI-QWWZWVQMSA-N
Looking for similar products? Visit Product Comparison Guide
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Amplification of dynamic chiral crown ether complexes during cyclic acetal formation.
Benzion Fuchs et al.
Angewandte Chemie (International ed. in English), 42(35), 4220-4224 (2003-09-23)
Timothy M Chapman et al.
Journal of the American Chemical Society, 127(2), 506-507 (2005-01-13)
Chlorination-elimination chemistry coupled with three-component Joullié-Ugi reaction and facile deprotection allowed efficient access to an array of polyhydroxylated pyrrolidines through parallel synthesis that may be considered to be a library of imino (aza) sugars (glycomimetics) and/or dihydroxyprolyl peptides (peptidomimetics). The
B J Ortwerth et al.
Experimental eye research, 58(6), 665-674 (1994-06-01)
L-Threose is a significant degradation product of ascorbic acid at pH 7.0 in the presence of oxygen. When compared to several other ascorbate-derived degradation products, it had the greatest ability to glycate and crosslink lens proteins in vitro. To determine
A Döss et al.
Physical review letters, 88(9), 095701-095701 (2002-02-28)
We have studied details of the molecular origin of slow secondary relaxation near T(g) in a series of neat polyalcohols by means of dielectric spectroscopy and (2)H NMR. From glycerol to threitol, xylitol, and sorbitol the appearance of the secondary
Justyna Wojno et al.
ACS chemical biology, 7(5), 847-855 (2012-02-14)
Invariant natural killer T (iNKT) cells are restricted by the non-polymorphic MHC class I-like protein, CD1d, and activated following presentation of lipid antigens bound to CD1d molecules. The prototypical iNKT cell agonist is α-galactosyl ceramide (α-GalCer). CD1d-mediated activation of iNKT
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service