392731
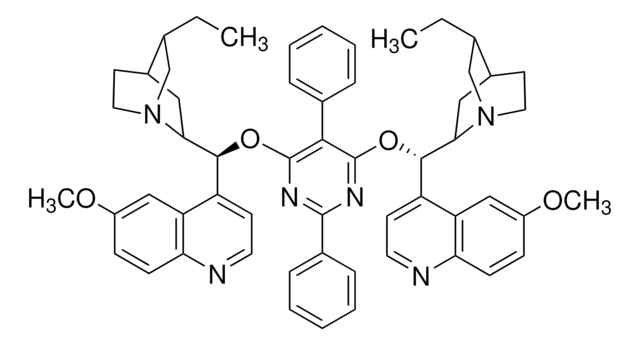
(DHQD)2PHAL
≥95%
Synonym(s):
Hydroquinidine 1,4-phthalazinediyl diether
About This Item
Recommended Products
assay
≥95%
form
powder
optical activity
[α]22/D −262°, c = 1.2 in methanol
greener alternative product score
old score: 5
new score: 3
Find out more about DOZN™ Scoring
greener alternative product characteristics
Waste Prevention
Less Hazardous Chemical Syntheses
Safer Solvents and Auxiliaries
Inherently Safer Chemistry for Accident Prevention
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
mp
160 °C (dec.) (lit.)
greener alternative category
SMILES string
CC[C@H]1CN2CCC1CC2[C@@H](Oc3nnc(O[C@H](C4CC5CCN4C[C@@H]5CC)c6ccnc7ccc(OC)cc67)c8ccccc38)c9ccnc%10ccc(OC)cc9%10
InChI
1S/C48H54N6O4/c1-5-29-27-53-21-17-31(29)23-43(53)45(35-15-19-49-41-13-11-33(55-3)25-39(35)41)57-47-37-9-7-8-10-38(37)48(52-51-47)58-46(44-24-32-18-22-54(44)28-30(32)6-2)36-16-20-50-42-14-12-34(56-4)26-40(36)42/h7-16,19-20,25-26,29-32,43-46H,5-6,17-18,21-24,27-28H2,1-4H3/t29-,30-,31-,32-,43+,44+,45-,46-/m0/s1
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
(DHQD)2PHAL is a modified cinchona alkaloid derivative mainly used as a ligand for enantioselective catalysis.[3]
Application
- Osmium trioxide catalyzed asymmetric dihydroxylation of olefins.[3]
- Palladium catalyzed Suzuki-Miyaura coupling of aryl/heteroaryl halides with aryl boronic acids in aqueous medium and in the absence of phosphine/organic solvent.[4]
- Copper(I)-catalyzed azide-alkyne cycloaddition reaction to synthesize 1,2,3-triazoles in water.[5]
Legal Information
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Related Content
The Sharpless Lab pursues useful new reactivity and general methods for selectively controlling chemical reactions.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service