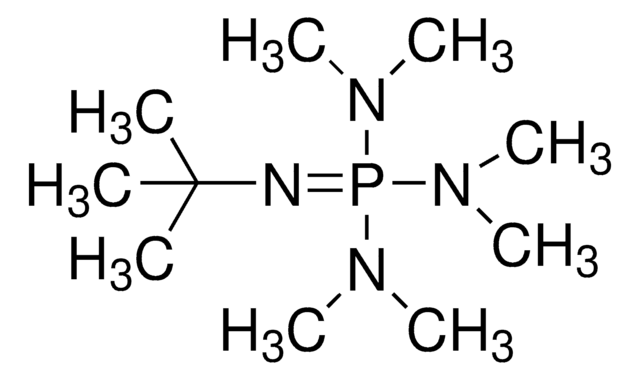
41541
1,8-Bis(tetramethylguanidino)naphthalene
≥98.0%
Synonym(s):
N′′,N′′′′′-1,8-Naphthalenediylbis(N,N,N′,N′-tetramethyl-guanidine), TMGN
Sign Into View Organizational & Contract Pricing
Select a Size
All Photos(1)
Select a Size
Change View
About This Item
Linear Formula:
C10H6[N=C[N(CH3)2]2]2
CAS Number:
Molecular Weight:
354.49
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
≥98.0%
impurities
≤0.5% water
mp
124-128 °C
solubility
toluene: 20 mg/mL, clear
functional group
amine
SMILES string
CN(C)\C(=N\c1cccc2cccc(\N=C(\N(C)C)N(C)C)c12)N(C)C
InChI
1S/C20H30N6/c1-23(2)19(24(3)4)21-16-13-9-11-15-12-10-14-17(18(15)16)22-20(25(5)6)26(7)8/h9-14H,1-8H3
InChI key
PSHHYQWGIGYWHP-UHFFFAOYSA-N
Related Categories
Analysis Note
may contain dark particles
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Dong Cao et al.
Talanta, 85(1), 345-352 (2011-06-08)
Determination of perfluorinated compounds (PFCs) is very important because of their potential hazards to the environment and human health. In present work, 1,8-bis (tetramethylguanidino)-naphthalene (TMGN), a superbasic proton sponge, was firstly employed as the matrix for quantitative detection of acidic
Strong Organic Bases as Building Blocks of Mesoporous Hybrid Catalysts for C-C Forming Bond Reactions.
Gianotti E, et al.
European Journal of Inorganic Chemistry, 32, 5175-5185 (2012)
Volker Raab et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 8(7), 1682-1693 (2002-04-05)
1,8-Bis(tetramethylguanidino)naphthalene (TMGN, 1) is a new, readily accessible, and stable "proton sponge" with an experimental pK(BH(+)) value of 25.1 in MeCN, which is nearly seven orders of magnitude higher in basicity than the classical proton sponge 1,8-bis(dimethylamino)-naphthalene (DMAN). Because of
Borislav Kovacević et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 8(7), 1694-1702 (2002-04-05)
The spatial and electronic structure of the very strong neutral organic bases bis(tetramethylguanidino)naphthalene (TMGN), 4,5-bis(tetramethylguanidino)fluorene (TMGF) and some related compounds are explored by ab initio computational methods. Their affinity towards the proton is scrutinized both in the gas phase and
Dong Cao et al.
The Analyst, 137(9), 2218-2225 (2012-03-22)
1,8-Bis(dimethylamino)naphthalene (DMAN), a classical 'proton sponge', was functionalized on silica particles as a novel solid-phase extraction (SPE) adsorbent (DMAN@silica) for extracting perfluoroalkyl sulfonates (PFSs). High reproducibility and excellent extraction capability for PFSs were obtained in a wide pH range (3.0~8.5).
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service

![1,5,7-Triazabicyclo[4.4.0]dec-5-ene 98%](/deepweb/assets/sigmaaldrich/product/structures/171/446/333d560c-cff6-4958-b489-5acfb3057cce/640/333d560c-cff6-4958-b489-5acfb3057cce.png)
![7-Methyl-1,5,7-triazabicyclo[4.4.0]dec-5-ene 98%](/deepweb/assets/sigmaaldrich/product/structures/237/769/028967ef-ca63-4f22-acc9-68f135a43b9a/640/028967ef-ca63-4f22-acc9-68f135a43b9a.png)
![1,8-Diazabicyclo[5.4.0]undec-7-ene 98%](/deepweb/assets/sigmaaldrich/product/structures/120/564/5b373e23-1624-489c-8efb-692de0f96ffb/640/5b373e23-1624-489c-8efb-692de0f96ffb.png)

![1,5-Diazabicyclo[4.3.0]non-5-ene 98%](/deepweb/assets/sigmaaldrich/product/structures/400/401/859b2474-712b-4448-b231-74d0bc3203f1/640/859b2474-712b-4448-b231-74d0bc3203f1.png)