All Photos(1)
About This Item
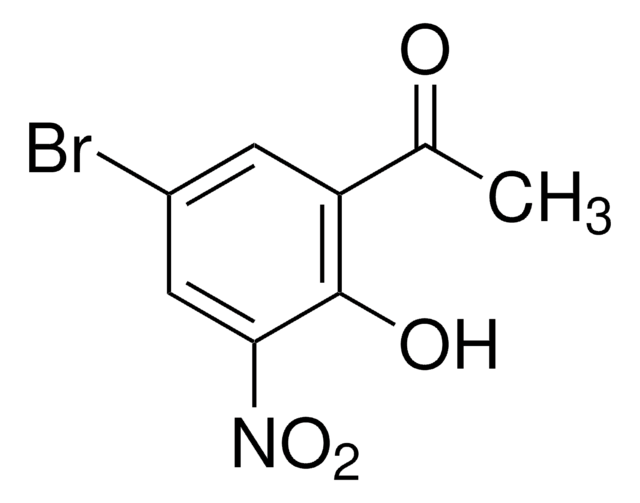
Linear Formula:
ClC6H2(OH)(NO2)COCH3
CAS Number:
Molecular Weight:
215.59
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
99%
mp
132-135 °C (lit.)
functional group
chloro
ketone
nitro
SMILES string
CC(=O)c1cc(Cl)cc(c1O)[N+]([O-])=O
InChI
1S/C8H6ClNO4/c1-4(11)6-2-5(9)3-7(8(6)12)10(13)14/h2-3,12H,1H3
InChI key
IUNBIQBAYUBIFD-UHFFFAOYSA-N
General description
5′-Chloro-2′-hydroxy-3′-nitroacetophenone (5-chloro-3-nitro-2-hydroxyacetophenone) is a nitro derivative of ortho-hydroxy aryl ketone. Its crystal structure has been studied by X-ray diffraction. It forms complex with 2-aminobenzoic acid (anthranilic acid), in which the nitro group is turned by approximately 40°C.
Application
5′-Chloro-2′-hydroxy-3′-nitroacetophenone may be used for the synthesis of 6-chloro-8-nitro-4-oxo-4H-chromene-3-carbaldehyde.
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Yoshinobu Ishikawa
Acta crystallographica. Section E, Structure reports online, 70(Pt 5), o547-o547 (2014-05-27)
In the title compound, C10H4ClNO5, the non-H atoms of the 6-chloro-chromone unit are coplanar (r.m.s. deviation = 0.017 Å) with the largest deviation from the mean plane [0.031 (2) Å] being found for the C=O C atom. The nitro group (NO2) is inclined
Phase transition and intramolecular hydrogen bonding in nitro derivatives of ortho-hydroxy acetophenones
Filarowski, A., et al.
Journal of Molecular Structure, 785(1), 7-13 (2006)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service