422843
Potassium trifluoromethanesulfonate
98%
Synonym(s):
Potassium triflate, Trifluoromethanesulfonic acid potassium salt
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
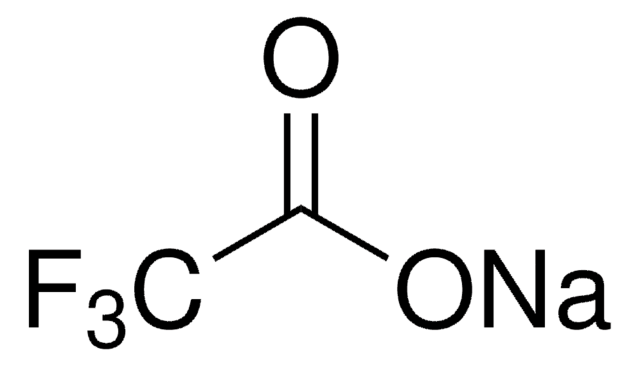
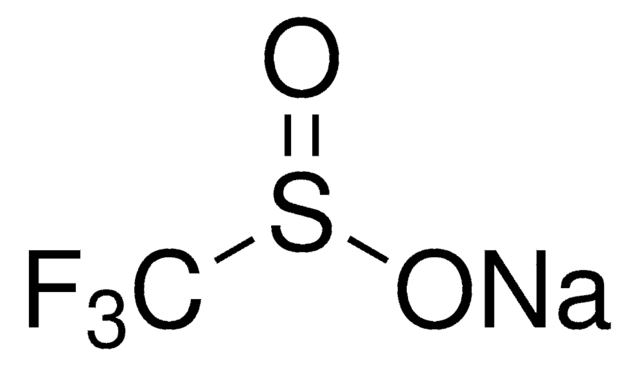
CF3SO3K
CAS Number:
Molecular Weight:
188.17
Beilstein/REAXYS Number:
3727495
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
98%
form
powder
functional group
fluoro
triflate
SMILES string
[K+].[O-]S(=O)(=O)C(F)(F)F
InChI
1S/CHF3O3S.K/c2-1(3,4)8(5,6)7;/h(H,5,6,7);/q;+1/p-1
InChI key
GLGXXYFYZWQGEL-UHFFFAOYSA-M
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Potassium trifluoromethanesulfonate (KOTf, potassium triflate) is the potassium salt of trifluoromethanesulphonic acid. It has been prepared by neutralizing a warm aqueous solution of trifluoromethanesulphonic acid with potassium carbonate. The structure of siloxane–poly(oxyethylene) hybrids doped with potassium triflate has been investigated.
Application
Potassium trifluoromethanesulfonate (potassium triflate) is suitable as a reagent in the synthesis of guanine-quadruplex hybrid materials by the self-assembly of a guanine-siloxane monomer.
It may be used in the following studies:
It may be used in the following studies:
- As a template source for constructing the heterotopic isothiosemicarbazide-based macrocyclic ligand.
- As a supporting electrolyte in the electrochemical study of evidence for gold anion in ethylenediamine.
- As a reagent in the synthesis of imidazolium salt, 3-methyl-1-(3R,3aR,6S,6aR)-[6-(benzyloxy)-hexahydrofuro[3,2-b]furan-3-yl]imidazolium trifluoromethanesulfonate.
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Potassium-controlled synthesis of heterotopic macrocycles based on isothiosemicarbazide.
Arion VB, et al.
Inorgorganica Chimica Acta, 328(1), 123-133 (2002)
Sara Santiago-Malagón et al.
Biosensors & bioelectronics, 175, 112879-112879 (2020-12-15)
One of the limitations of many skin-patch wearable sensors today is their dependence on silicon-based electronics, increasing their complexity and unit cost. Self-powered sensors, in combination with electrochromic materials, allow simplifying the construction of these devices, leading to powerful analytical
Properties and reactivities of pentadentate ethylenediaminetetraacetate complexes of ruthenium (III) and-(II).
Matsubara T and Creutz C.
Inorganic Chemistry, 18(7), 1956-1966 (1979)
Gregory D Bowden et al.
Scientific reports, 9(1), 11370-11370 (2019-08-08)
Recent advancements in 18F radiochemistry, such as the advent of copper-mediated radiofluorination (CMRF) chemistry, have provided unprecedented access to novel chemically diverse PET probes; however, these multicomponent reactions have come with a new set of complex optimization problems. Design of
Evidence for the gold anion in ethylenediamine.
Jagannathan R, et al.
Inorganic Chemistry, 24(1), 113-114 (1985)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service