All Photos(1)
About This Item
Linear Formula:
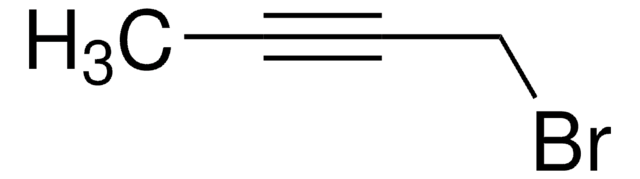
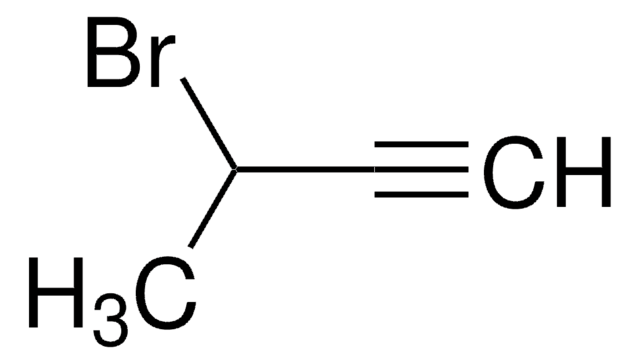
C2H5C≡CCH2Br
CAS Number:
Molecular Weight:
147.01
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
97%
form
liquid
refractive index
n20/D 1.498 (lit.)
bp
93-94 °C/113 mmHg (lit.)
density
1.438 g/mL at 25 °C (lit.)
functional group
alkyl halide
bromo
SMILES string
CCC#CCBr
InChI
1S/C5H7Br/c1-2-3-4-5-6/h2,5H2,1H3
Inchi Key
VDHGRVFJBGRHMD-UHFFFAOYSA-N
Related Categories
General description
1-Bromo-2-pentyne is an halogenated hydrocarbon.
Application
1-Bromo-2-pentyne may be employed for the following syntheses:
- stereochemically restricted lactone-type analogs of jasmonic acids, 5-oxa-7-epi-jasmonic acid and 5-oxa-jasmonic acid
- 4,7-decadienal, 4,7-tridecadienal, 5,8-tetradecadienal and 6,9-dodecadienal (all-cis)
- 5-ethyl-4-methylene-6-phenyl-3a,4,7,7a-tetrahydroisobenzofuran-1,3-dione
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Flam. Liq. 3 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
3 - Flammable liquids
wgk_germany
WGK 3
flash_point_f
105.8 °F - closed cup
flash_point_c
41 °C - closed cup
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthesis of some aliphatic dienals.
Ward JP and Van Dorp DA.
Rec. Trav. Chim., 88(2), 177-184 (1969)
Synthesis of Cyclic Compounds Having exo-Methylene Groups through the Diels-Alder Reactions of Vinyl Allenes Obtained from Propargyl Bromide and Indium.
Lee K and Lee PH.
Bull. Korean Chem. Soc., 29(2), 487-487 (2008)
H Toshima et al.
Bioscience, biotechnology, and biochemistry, 64(9), 1988-1992 (2000-10-31)
5-Oxa-7-epi-jasmonic acid and 5-oxa-jasmonic acid, which are stereochemically restricted lactone-type analogues of jasmonic acids, were synthesized via three-component coupling of 2(5H)-furanone, tert-butyl acetate and 1-bromo-2-pentyne. After acidic deprotection of the tert-butyl esters, the (Z)-olefin was introduced by catalytic partial reduction
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service