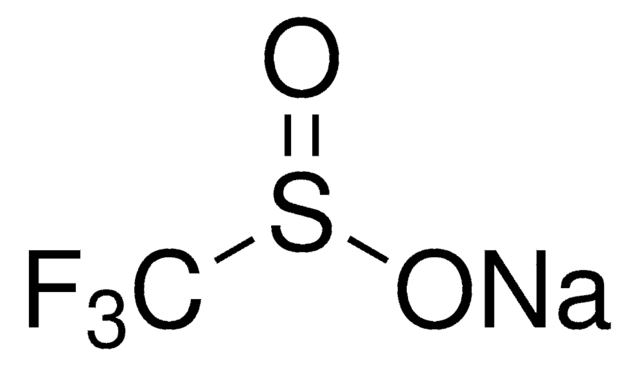
433063
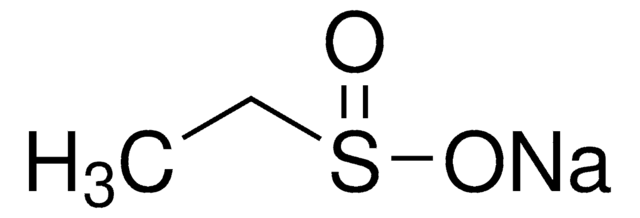
Sodium methanesulfinate
technical grade, 85%
Synonym(s):
Methanesulfinic acid sodium salt
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
CH3SO2Na
CAS Number:
Molecular Weight:
102.09
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
grade
technical grade
Quality Level
assay
85%
mp
222-226 °C (dec.) (lit.)
functional group
sulfinic acid
SMILES string
[Na+].CS([O-])=O
InChI
1S/CH4O2S.Na/c1-4(2)3;/h1H3,(H,2,3);/q;+1/p-1
InChI key
LYPGDCWPTHTUDO-UHFFFAOYSA-M
Looking for similar products? Visit Product Comparison Guide
General description
Sodium methanesulfinate is an aliphatic sodium sulfinate. Conjugate addition of sodium methanesulfinate to vinyl heterocycles has been described. Cross-coupling reaction between aryl boronic acid and sodium methanesulfinate has been studied. Its stock solution was prepared from methanesulfonic acid by adding one equivalent of sodium hydroxide and diluting it to 4M.
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
C F Babbs et al.
Free radical biology & medicine, 6(5), 493-503 (1989-01-01)
A major impediment to the confirmation of free radical mechanisms in pathogenesis is a lack of direct, chemical evidence that oxygen centered free radicals actually arise in living tissues in quantities sufficient to cause serious damage. This investigation was conducted
Conjugate addition of sodium methanesulfinate to vinyl pyridines and diazines for the synthesis of aliphatic sulfones.
Schaaf GM, et al.
Tetrahedron Letters, 50(17), 1928-1933 (2009)
Matthew L Dawson et al.
Proceedings of the National Academy of Sciences of the United States of America, 109(46), 18719-18724 (2012-10-24)
Airborne particles affect human health and significantly influence visibility and climate. A major fraction of these particles result from the reactions of gaseous precursors to generate low-volatility products such as sulfuric acid and high-molecular weight organics that nucleate to form
Cecilia Arsene et al.
Environmental science & technology, 36(23), 5155-5163 (2003-01-14)
Dimethyl sulfoxide (CH3S(O)CH3: DMSO) is an important product of dimethyl sulfide (CH3SCH3: DMS) photooxidation. The mechanism of the OH-radical initiated oxidation of DMSO is still highly uncertain and a major aim of recent studies has been to establish if methane
One-pot synthesis of aryl sulfoxides and sulfonium salts from sulfinic acid as a novel sulfurizing agent.
Yamamoto K, et al.
Chemical Communications (Cambridge, England), 17, 2099-2100 (1996)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service


![1,8-Diazabicyclo[5.4.0]undec-7-ene 98%](/deepweb/assets/sigmaaldrich/product/structures/120/564/5b373e23-1624-489c-8efb-692de0f96ffb/640/5b373e23-1624-489c-8efb-692de0f96ffb.png)