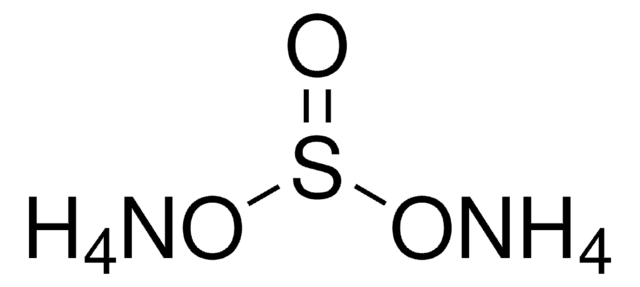
455849
Ammonium hydrogensulfate
99.99% trace metals basis
Synonym(s):
Ammonium bisulfate, Ammonium sulfate monobasic
About This Item
Recommended Products
assay
99.99% trace metals basis
form
crystalline
impurities
≤200 mg/kg Trace metallic impurities analysis (ICP)
bp
350 °C (dec.)(lit.)
mp
121-145 °C (lit.)
density
1.79 g/mL at 25 °C (lit.)
SMILES string
N.OS(O)(=O)=O
InChI
1S/H3N.H2O4S/c;1-5(2,3)4/h1H3;(H2,1,2,3,4)
InChI key
BIGPRXCJEDHCLP-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
- Enhancement of Aesthetic Dental CAD-CAM Materials through Surface Etching with a Mixed Aqueous Solution of Ammonium Fluoride and Ammonium Hydrogen Sulfate - This study explores the potential of ammonium hydrogen sulfate in surface etching applications for dental materials, focusing on its low toxicity and effective etching capabilities (Y Nishizawa et al., 2024).
- Thermodynamics of ammonioalunite precipitation in ammonium aluminum sulfate solution - Investigates the thermodynamic properties of ammonium aluminum sulfate solutions, providing insights into chemical processes involving ammonium hydrogen sulfate (X Yang et al., 2020).
- Hygroscopic behavior and chemical composition evolution of internally mixed aerosols composed of oxalic acid and ammonium sulfate - Studies the hygroscopic properties of mixed aerosol particles, including those formed with ammonium hydrogen sulfate, to understand atmospheric chemical processes better (X Wang et al., 2017).
signalword
Danger
hcodes
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1B
Storage Class
8B - Non-combustible corrosive hazardous materials
wgk_germany
WGK 1
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service