481122
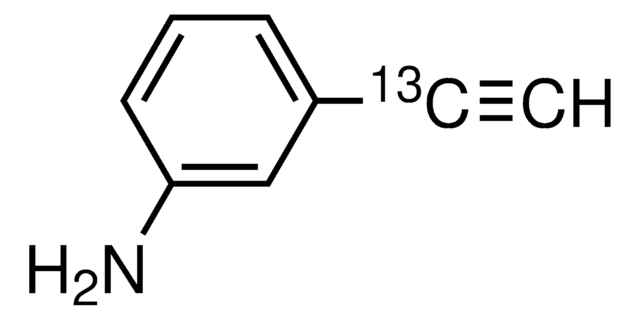
4-Ethynylaniline
97%
Synonym(s):
1-Amino-4-ethynylbenzene, P-APAC
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
HC≡CC6H4NH2
CAS Number:
Molecular Weight:
117.15
Beilstein/REAXYS Number:
2205181
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
97%
reaction suitability
reaction type: click chemistry
mp
98-102 °C (dec.) (lit.)
SMILES string
Nc1ccc(cc1)C#C
InChI
1S/C8H7N/c1-2-7-3-5-8(9)6-4-7/h1,3-6H,9H2
InChI key
JXYITCJMBRETQX-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
4-Ethynylaniline, also known as p-ethynylaniline, is a terminal alkyne. Its synthesis using 2-methyl-3-butyn-2-ol (MEBYNOL) has been reported. The transition metal catalyzed polymerization of 4-ethynylaniline to afford poly(4-ethynylaniline) has been reported. The impact of the surface functionalization with 4-ethynylaniline on the thermal behavior of multi-walled carbon nanotubes (MWNTs) and graphene has been investigated.
Application
4-Ethynylaniline may be used in the synthesis of N-methyliminodiethyl 4-(4-ethynylphenyliminomethyl)benzeneboronate. It can also be used to prepare an acetylene ligand, HC2-NDI (NDI= 1,4,5,8-naphthalenediimide).
Used as an alkyne component in a synthesis of indoles from nitroarenes in the presence of a palladium-phenantroline catalyst.
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Platinum (II) phosphine complexes with acetylene ligands containing 1,4,5,8-naphthalenediimide: Synthesis, crystal structure and electrochemistry.
Shavaleev NM, et al.
Journal of Organometallic Chemistry, 692(4), 921-925 (2007)
Fabio Ragaini et al.
The Journal of organic chemistry, 71(10), 3748-3753 (2006-05-06)
Palladium-phenanthroline complexes efficiently catalyze the reaction of nitroarenes with arylalkynes and CO to give 3-arylindoles by an ortho-C-H functionalization of the nitroarene ring. Both electron-withdrawing and electron-donating substituents are tolerated on the nitroarene, except for bromide and activated chloride. Nitroarenes
A simple and economical synthetic route to p-ethynylaniline and ethynyl-terminated substrates.
Melissaris AP and Litt MH.
The Journal of Organic Chemistry, 59(19), 5818-5821 (1994)
Synthesis and Electro-Optical Properties of Poly(4-ethynylaniline).
Gui TL, et al.
Mol. Cryst. Liq. Cryst., 459(1), 19-299 (2006)
Comparative study of the covalent diazotization of graphene and carbon nanotubes using thermogravimetric and spectroscopic techniques.
Castelain M, et al.
Physical Chemistry Chemical Physics, 15(39), 16806-16811 (2013)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service



![4-[(4-Fluorophenyl)ethynyl]phenol AldrichCPR](/deepweb/assets/sigmaaldrich/product/structures/188/684/0b16c024-0d26-4b43-a607-60b40446e593/640/0b16c024-0d26-4b43-a607-60b40446e593.png)