522864
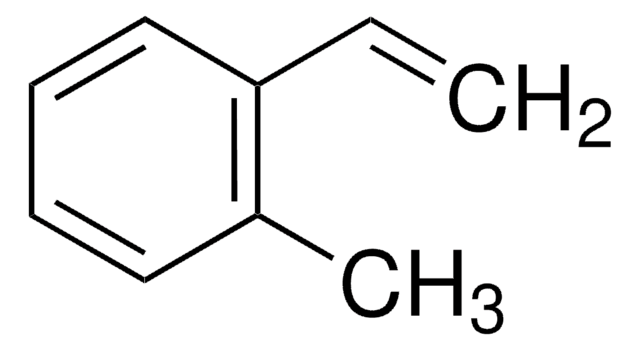
Methylstyrene
60% meta, 40% para and 1% ortho, 99%, contains ~50 ppm 4-tert-butylcatechol as inhibitor
Synonym(s):
Vinyltoluene
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
CH3C6H4CH=CH2
CAS Number:
Molecular Weight:
118.18
Beilstein/REAXYS Number:
1209317
EC Number:
MDL number:
UNSPSC Code:
12162002
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
Quality Level
assay
99%
contains
~50 ppm 4-tert-butylcatechol as inhibitor
refractive index
n20/D 1.5425 (lit.)
bp
168 °C (lit.)
density
0.893 g/mL at 25 °C (lit.)
storage temp.
2-8°C
SMILES string
Cc1ccc(C=C)cc1.Cc2cccc(C=C)c2
InChI
1S/2C9H10/c1-3-9-6-4-8(2)5-7-9;1-3-9-6-4-5-8(2)7-9/h2*3-7H,1H2,2H3
InChI key
VAPKHDZBJXRVNG-UHFFFAOYSA-N
Related Categories
General description
Methylstyrene can be used as a monomer in the synthesis of block copolymers for resins and adhesives. It is widely used as a starting material to prepare latexes for paper coating applications.
Application
- Applications in cancer therapy: Investigation into (−)-Epicatechin incorporated in phytopharmaceuticals for cancer treatment discusses its bioactive properties and synergistic effects when combined with other therapeutic agents, providing new avenues for integrative cancer therapies (Idoudi et al., 2024).
signalword
Danger
Hazard Classifications
Acute Tox. 4 Inhalation - Asp. Tox. 1 - Eye Irrit. 2 - Flam. Liq. 3 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
3 - Flammable liquids
wgk_germany
WGK 3
flash_point_f
125.6 °F - closed cup
flash_point_c
52 °C - closed cup
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
J A Cohen et al.
The journal of physical chemistry. B, 113(12), 3709-3714 (2009-03-07)
We present a phenomenological one-parameter scaling equation of state that accurately represents osmotic pressures of neutral flexible polymers in good solvents from the dilute through the semidilute regime. The equation comprises a sum of scaled van't Hoff and des Cloizeaux
Christopher P Burke et al.
The Journal of organic chemistry, 72(16), 6320-6323 (2007-07-12)
Asymmetric epoxidation of various olefins with an N-aryl-substituted oxazolidinone-containing ketone as catalyst and hydrogen peroxide as the primary oxidant has been investigated, and up to 96% ee was obtained.
M Groner et al.
Marine pollution bulletin, 42(10), 935-941 (2001-11-06)
The chemical identities of several organic compounds that dominate the ultraviolet (UV) fluorescence of water after exposure to gasoline, diesel fuel and crude oil are presented. A combination of high-performance liquid chromatography with UV-fluorescence detection, fluorescence spectroscopy and gas chromatography-mass
Jay A Grobler et al.
Proceedings of the National Academy of Sciences of the United States of America, 99(10), 6661-6666 (2002-05-09)
The process of integrating the reverse-transcribed HIV-1 DNA into the host chromosomal DNA is catalyzed by the virally encoded enzyme integrase (IN). Integration requires two metal-dependent reactions, 3' end processing and strand transfer. Compounds that contain a diketo acid moiety
Henry Dube et al.
Journal of the American Chemical Society, 132(29), 9984-9985 (2010-07-09)
The trans to cis isomerization of 4,4'-dimethylazobenzene by the nonchemical stimuli light and heat alters its guest binding properties, allowing control over encapsulation of a second guest. We show here how this remote control for reversible encapsulation can be used
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service