All Photos(1)
About This Item
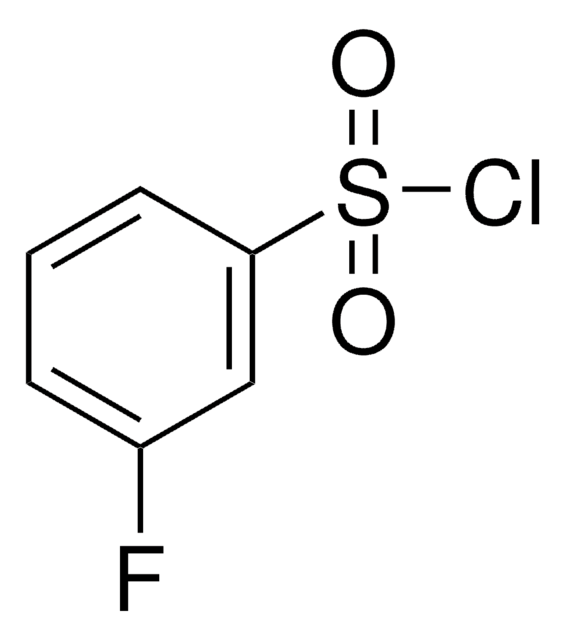
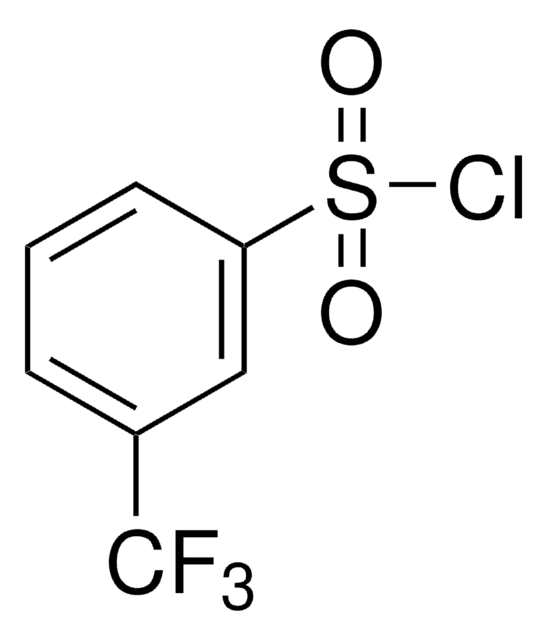
Linear Formula:
FC6H4SO2Cl
CAS Number:
Molecular Weight:
194.61
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
97%
refractive index
n20/D 1.537 (lit.)
bp
246-247 °C (lit.)
mp
27-30 °C (lit.)
density
1.47 g/mL at 25 °C (lit.)
functional group
fluoro
SMILES string
Fc1ccccc1S(Cl)(=O)=O
InChI
1S/C6H4ClFO2S/c7-11(9,10)6-4-2-1-3-5(6)8/h1-4H
InChI key
ZSZKAQCISWFDCQ-UHFFFAOYSA-N
General description
2-Fluorobenzenesulfonyl chloride, also known as o-fluorobenzenesulfonyl chloride, is a flurinated arylsulfonyl chloride. It can be prepared from o-benzenedisulfonyl fluoride.
Application
2-Fluorobenzenesulfonyl chloride may be used in the preparation of the following furan derivatives:
It may also be used to prepare:
- 2-(2-fluorophenyl)benzofuran
- 2-butyl-5-(2-fluorophenyl)furan
- 2-(2-fluorophenyl)-3,6-dimethyl-4,5,6,7-tetrahydrobenzofuran
It may also be used to prepare:
- 2-fluorobenzenesulfonamide
- methyl 2-{[(2-fluorophenyl)sulfonyl]amino}-5,6,7,8-tetrahydro-1-naphthalenecarboxylate
- potassium fluorobenzene-2-sulfonate
- 1-(2-bromobenzyl)-2-(2-fluorophenyl)pyrrole
signalword
Danger
hcodes
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1B
Storage Class
8A - Combustible corrosive hazardous materials
wgk_germany
WGK 3
flash_point_f
230.0 °F - closed cup
flash_point_c
110 °C - closed cup
ppe
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Regiocontroled Palladium?Catalysed Direct Arylation at Carbon C2 of Benzofurans using Benzenesulfonyl Chlorides as the Coupling Partners.
Loukotova L, et al.
ChemCatChem, 6(5), 1303-1309 (2014)
Water-Soluble Phosphines. 6.1 Tailor-Made Syntheses of Chiral Secondary and Tertiary Phosphines with Sulfonated Aromatic Substituents: Structural and Quantum Chemical Studies.
Bitterer F, et al.
Inorganic Chemistry, 35(14), 4103-4113 (1996)
Discovery and optimization of anthranilic acid sulfonamides as inhibitors of methionine aminopeptidase-2: a structural basis for the reduction of albumin binding.
Sheppard GS, et al.
Journal of Medicinal Chemistry, 49(13), 3832-3849 (2006)
Benzenesulfonyl Chlorides: Alternative Coupling Partners for Regiocontrolled Palladium-Catalyzed Direct Desulfitative 5-Arylation of Furans.
Beladhria A, et al.
Synthesis, 46(18), 2515-2523 (2014)
Potassium fluoride catalyzed fluorodesulfonylations of aryl sulfonyl fluorides.
Van der Puy M.
The Journal of Organic Chemistry, 53(18), 4398-4401 (1988)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service