542865
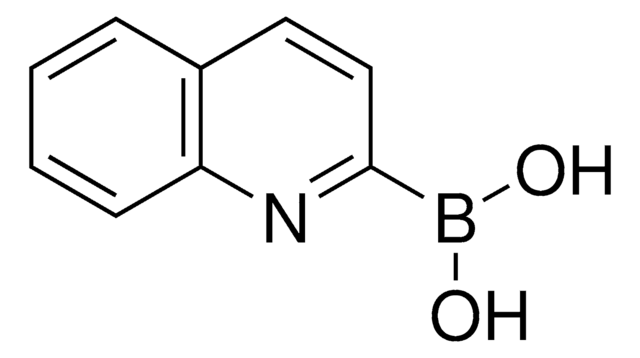
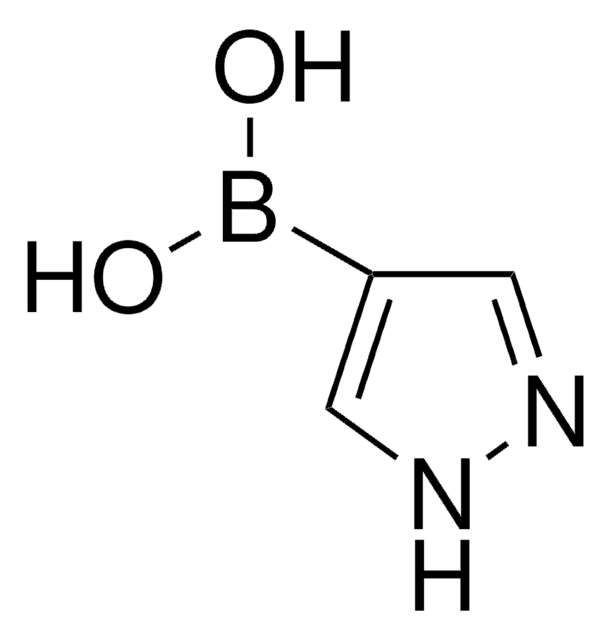
8-Quinolinylboronic acid
technical grade
Synonym(s):
8-Quinolineboronic acid
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C9H8BNO2
CAS Number:
Molecular Weight:
172.98
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
grade
technical grade
Quality Level
form
solid
mp
>300 °C (lit.)
SMILES string
OB(O)c1cccc2cccnc12
InChI
1S/C9H8BNO2/c12-10(13)8-5-1-3-7-4-2-6-11-9(7)8/h1-6,12-13H
InChI key
KXJJSKYICDAICD-UHFFFAOYSA-N
Application
Reactant involved in:
- C-H and C-S bond activations
- Synthesis of pyridazine via sequential amination / Suzuki coupling / alkylation reactions
- Suzuki-Miyaura coupling reactions for synthesis of biaryl monophosphorus ligands, fused tricyclic oxa-quinolones, or substituted β-amino acids
- Copper-catalyzed azidation with sodium azide
- Studies of the affect of fluoride on the stability of boronic acids during click reactions
Other Notes
Contains varying amounts of anhydride
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Mayoorini Majuran et al.
ChemPlusChem, 85(2), 346-352 (2020-02-07)
We report the synthesis, photophysics, electrochemistry and electrochemiluminescence (ECL) of two dqp (dqp=2,6-di(quinoline-8-yl)pyridine) based ruthenium(II) complexes, bearing either a n-butyl ester (1) or the corresponding carboxylic acid functionality (2). The complexes were prepared from [Ru(dqp)(MeCN)3 ][PF6 ]2 by reaction with
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service