All Photos(1)
About This Item
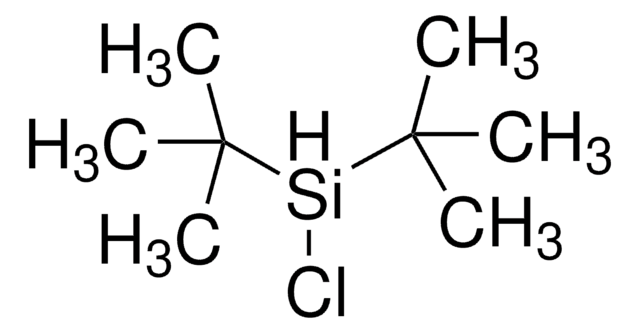
Linear Formula:
[(CH3)3C]2SiH2
CAS Number:
Molecular Weight:
144.33
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
97%
refractive index
n20/D 1.42 (lit.)
bp
129-130 °C (lit.)
mp
−38 °C (lit.)
density
0.729 g/mL at 25 °C (lit.)
SMILES string
[H][Si]([H])(C(C)(C)C)C(C)(C)C
InChI
1S/C8H20Si/c1-7(2,3)9-8(4,5)6/h9H2,1-6H3
InChI key
ZLKSBZCITVUTSN-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Di-tert-butylsilane can be used as a reagent to synthesize:
- 1,1-di-tert-butyl-N-phenylsilanamine by dehydrogenative coupling with aniline in the presence of supported gold catalyst.
- Benzyloxy di-tert-butylsilane by dehydrocoupling of benzyl alcohol using NaOH as a catalyst.
- Di-tert-butyl(3-cyclohexylprop-1-yn-1-yl)silane by silylation reaction with 2-propyn-1-ylcyclohexane using alkali metal hydroxide as a catalyst.
signalword
Danger
hcodes
Hazard Classifications
Flam. Liq. 2
Storage Class
3 - Flammable liquids
wgk_germany
WGK 3
flash_point_f
28.0 °F - closed cup
flash_point_c
-2.22 °C - closed cup
ppe
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Alkali metal-hydroxide-catalyzed C (sp)-H bond silylation
Toutov AA, et al.
Journal of the American Chemical Society, 139(4), 1668-1674 (2017)
Dehydrogenative Coupling of Hydrosilanes with Amines Using Au/HAP
Uozumi Y and Hamasaka G
Synfacts, 11(05), 0558-0558 (2015)
Sodium Hydroxide Catalyzed Dehydrocoupling of Alcohols with Hydrosilanes
Toutov AA, et al.
Organic Letters, 18(22), 5776-5779 (2016)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service