549983
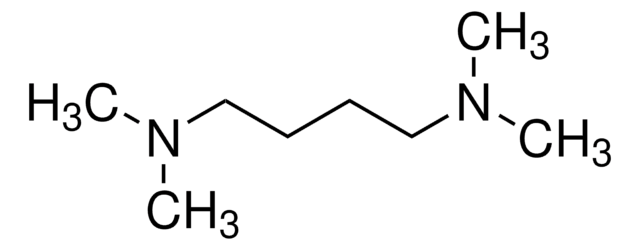
N,N,N′,N′-Tetramethyl-1,3-propanediamine
≥99%
Synonym(s):
1,3-Bis(dimethylamino)propane, TMPDA
About This Item
Recommended Products
vapor pressure
760 mmHg ( 145 °C)
assay
≥99%
refractive index
n20/D 1.4234 (lit.)
bp
145-146 °C (lit.)
density
0.779 g/mL at 25 °C (lit.)
functional group
amine
SMILES string
CN(C)CCCN(C)C
InChI
1S/C7H18N2/c1-8(2)6-5-7-9(3)4/h5-7H2,1-4H3
InChI key
DMQSHEKGGUOYJS-UHFFFAOYSA-N
Gene Information
rat ... Grin2a(24409)
Looking for similar products? Visit Product Comparison Guide
General description
- As a catalyst for the Baylis-Hillman reaction of cycloalkenones.
- In the preparation of homodimeric asymmetric monomethine cyanine dyes during the bisquaternization process.
- As a ligand (L) for the preparation of dinuclear μ-carbonato-dicopper(II) species.
Application
- Deaggregation of Zinc Dihydride by Lewis Acids Including Carbon Dioxide in the Presence of Nitrogen Donors.: This study explores the deaggregation of zinc dihydride using Lewis acids such as carbon dioxide in the presence of nitrogen donors, including N,N,N′,N′-Tetramethyl-1,3-propanediamine, highlighting its application in organic synthesis and catalyst development (Ritter et al., 2021).
- Contribution of Cross-Linker and Silica Morphology on Cr(VI) Sorption Performances of Organic Anion Exchangers Embedded into Silica Pores.: This research focuses on the sorption performances of Cr(VI) using organic anion exchangers embedded in silica pores, demonstrating the role of N,N,N′,N′-Tetramethyl-1,3-propanediamine in enhancing cross-linking and silica morphology for improved sorption efficiency (Dragan & Humelnicu, 2020).
- Nanopore-induced host-guest charge transfer phenomena in a metal-organic framework.: The study investigates host-guest charge transfer phenomena within nanopores of a metal-organic framework, using N,N,N′,N′-Tetramethyl-1,3-propanediamine as a key component in the framework′s design, demonstrating its significance in materials science and nanoengineering (Yamamoto et al., 2018).
- Cross-Linking of a Hydrophilic, Antimicrobial Polycation toward a Fast-Swelling, Antimicrobial Superabsorber and Interpenetrating Hydrogel Networks with Long Lasting Antimicrobial Properties.: This paper details the development of a hydrophilic, antimicrobial polycation cross-linked with N,N,N′,N′-Tetramethyl-1,3-propanediamine to create a fast-swelling, antimicrobial superabsorber and hydrogel network with prolonged antimicrobial effects (Strassburg et al., 2017).
- CO(2)-Switchable microemulsion based on a pseudogemini surfactant.: The research presents a CO2-switchable microemulsion system based on a pseudogemini surfactant incorporating N,N,N′,N′-Tetramethyl-1,3-propanediamine, showcasing its potential in responsive material systems for environmental applications (Liu et al., 2017).
Legal Information
signalword
Danger
Hazard Classifications
Acute Tox. 3 Inhalation - Acute Tox. 4 Dermal - Acute Tox. 4 Oral - Aquatic Chronic 2 - Eye Dam. 1 - Flam. Liq. 3 - Skin Corr. 1B - STOT SE 3
target_organs
Respiratory system
Storage Class
3 - Flammable liquids
wgk_germany
WGK 2
flash_point_f
87.8 °F - Equilibrium method
flash_point_c
31 °C - Equilibrium method
ppe
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service

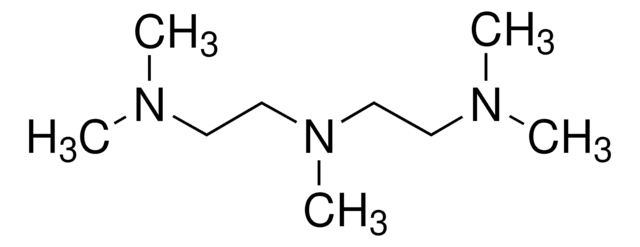
![Bis[2-(N,N-dimethylamino)ethyl] ether 97%](/deepweb/assets/sigmaaldrich/product/structures/372/323/505a46ae-b067-4177-8e5f-19a3f4ef9c44/640/505a46ae-b067-4177-8e5f-19a3f4ef9c44.png)