All Photos(1)
About This Item
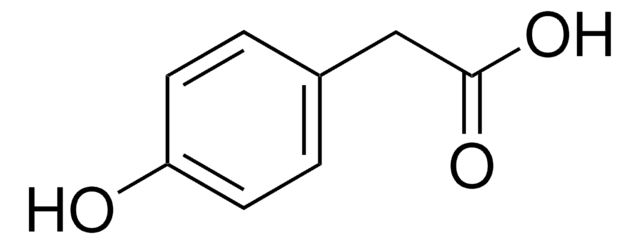
Empirical Formula (Hill Notation):
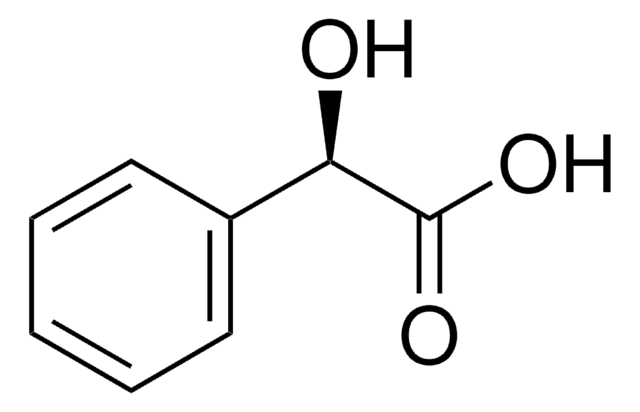
C8H8O4
CAS Number:
Molecular Weight:
168.15
Beilstein/REAXYS Number:
2365378
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
≥97.0% (T)
mp
128-132 °C
functional group
carboxylic acid
hydroxyl
SMILES string
OC(C(O)=O)c1cccc(O)c1
InChI
1S/C8H8O4/c9-6-3-1-2-5(4-6)7(10)8(11)12/h1-4,7,9-10H,(H,11,12)
InChI key
OLSDAJRAVOVKLG-UHFFFAOYSA-N
General description
3-Hydroxymandelic acid is a hydroxy acid derivative. Chiral separation of 3-hydroxymandelic acid has been achieved by ligand-exchange capillary electrochromatography.
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
J M Midgley et al.
Biomedical mass spectrometry, 6(11), 485-490 (1979-11-01)
o-Hydroxymandelic acid and m-hydroxymandelic acid have been identified in human urine by gas chromatography mass spectrometry selected ion monitoring. After solvent extraction the urinary acids were converted to their O-trifluoroacetoxy methyl ester derivatives which were identified by comparison of the
K E Ibrahim et al.
The Journal of pharmacy and pharmacology, 35(3), 144-147 (1983-03-01)
The metabolism of R-(-)-m-synephrine (administered orally and by inhalation in man and intraperitoneally in rats) was studied quantitatively by a gas chromatography-mass spectrometry-selected ion monitoring (g.c.-m.s.-s.i.m.) method using deuterated internal standards. When m-synephrine hydrochloride was administered orally to humans in
M W Couch et al.
Clinica chimica acta; international journal of clinical chemistry, 158(1), 109-114 (1986-07-15)
The urinary concentrations of o-hydroxymandelic acid, m-hydroxymandelic acid, p-hydroxymandelic acid, homovanillic acid and vanillylmandelic acid were determined in 57 healthy children and 9 patients with neuroblastoma. The concentrations of o-hydroxymandelic acid and p-hydroxymandelic were not significantly different for both groups
Increased excretion of m-Hydroxyphenylglycol and m-Hydroxymandelic acid in neuroblastoma.
C M Williams et al.
Biochemical medicine, 28(3), 305-309 (1982-12-01)
Role of the charge in continuous beds in the chiral separation of hydroxy acids by ligand-exchange capillary electrochromatography.
Lecnik O, et al.
Electrophoresis, 24(17), 2983-2985 (2003)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service