608297
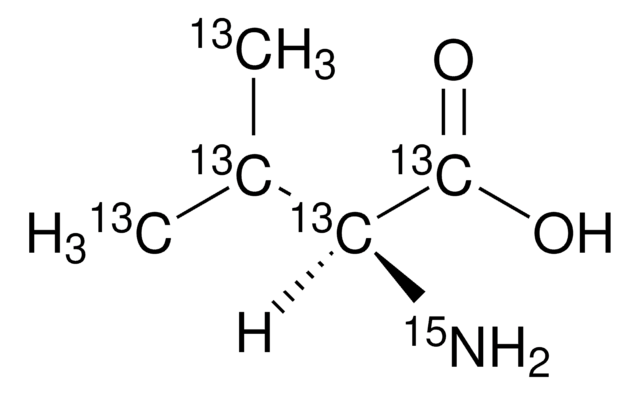
ISOGRO®-13C,15N,D Powder -Growth Medium
98 atom % 15N, 97-99 atom % D, 99 atom % 13C
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Recommended Products
isotopic purity
99 atom % 13C
98 atom % 15N
97-99 atom % D
form
solid
technique(s)
bio NMR: suitable
protein expression: suitable
storage temp.
−20°C
Related Categories
General description
ISOGRO® media is required for overcoming the growth limitations of minimal media. ISOGRO products are lysates of algae grown with stable isotopes (13C, 15N, and/or D). ISOGRO®-13C,15N,D powder -growth medium gives uniform labeling for protein expression NMR (nuclear magnetic resonance) studies.
A typical algal lysate (ISOGRO medium) contains: 30% salts, 3% water, 2% glucose and 65% amino acids/peptides.
A typical algal lysate (ISOGRO medium) contains: 30% salts, 3% water, 2% glucose and 65% amino acids/peptides.
Application
ISOGRO®-13C,15N,D Powder -Growth Medium has been used for the generation of isotopically labeled recombinant tau to identify multiple phosphorylations in tau by NMR (nuclear magnetic resonance) spectroscopy.
Packaging
This product may be available from bulk stock and can be packaged on demand. For information on pricing, availability and packaging, please contact Stable Isotopes Customer Service.
Legal Information
ISOGRO is a registered trademark of Merck KGaA, Darmstadt, Germany
Storage Class
11 - Combustible Solids
wgk_germany
WGK 1
flash_point_f
Not applicable
flash_point_c
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Nuclear Magnetic Resonance Spectroscopy for the Identification of Multiple Phosphorylations of Intrinsically Disordered Proteins.
Danis C, et al.
Journal of Visualized Experiments, 118, doi: 10-doi: 10 (2016)
Ara Celi DiCostanzo et al.
The Journal of biological chemistry, 287(33), 27997-28006 (2012-06-29)
Light chain amyloidosis is an incurable protein misfolding disease where monoclonal immunoglobulin light chains misfold and deposit as amyloid fibrils, causing organ failure and death. Previously, we determined that amyloidogenic light chains AL-09 and AL-103 do not form fibrils at
Nicholas C Fitzkee et al.
The Journal of biological chemistry, 285(23), 18072-18084 (2010-04-07)
The human immunodeficiency virus type 1 (HIV-1) integrase (IN) is a critical enzyme involved in infection. It catalyzes two reactions to integrate the viral cDNA into the host genome, 3' processing and strand transfer, but the dynamic behavior of the
J L Urbauer et al.
The Journal of biological chemistry, 276(44), 41128-41132 (2001-08-24)
The association of the bacteriophage T4-encoded AsiA protein with the final sigma(70) subunit of the Escherichia coli RNA polymerase is one of the principal events governing transcription of the T4 genome. Analytical ultracentrifugation and NMR studies indicate that free AsiA
Alexandria N Richart et al.
The Journal of biological chemistry, 287(22), 18730-18737 (2012-04-12)
The chromoshadow domain (CSD) of heterochromatin protein 1 (HP1) was recently shown to contribute to chromatin binding and transcriptional regulation through interaction with histone H3. Here, we demonstrate the structural basis of this interaction for the CSD of HP1α. This
Articles
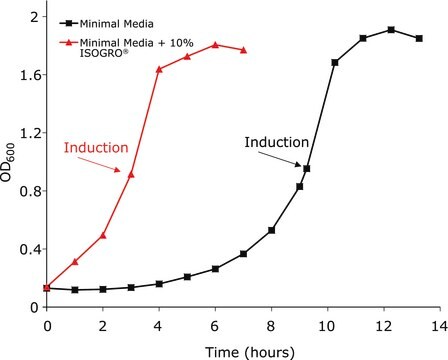
Utilizing ISOGRO® Supplementation of M9 Minimal Media to Enhance Recombinant Protein Expression.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service