649694
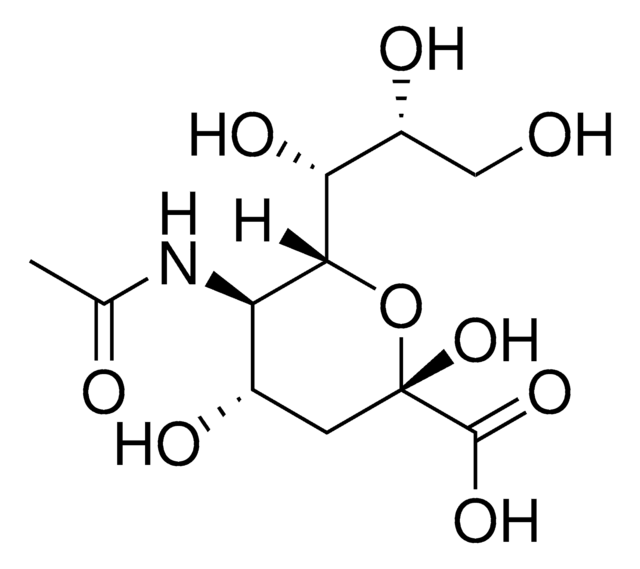
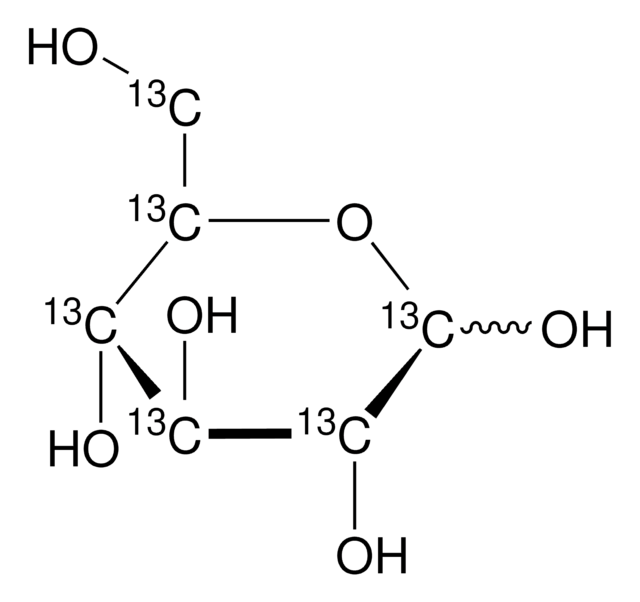
N-Acetyl-D-neuraminic acid-1,2,3-13C3
≥99 atom % 13C, ≥97% (CP)
Synonym(s):
Sialic-13C3 acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
13C3C8H19NO9
CAS Number:
Molecular Weight:
312.25
MDL number:
UNSPSC Code:
12352005
PubChem Substance ID:
NACRES:
NA.12
Recommended Products
isotopic purity
≥99 atom % 13C
Quality Level
assay
≥97% (CP)
mp
186 °C (lit.)
mass shift
M+3
storage temp.
−20°C
SMILES string
CC(=O)N[C@@H]1[C@@H](O)[13CH2][13C](O)(O[C@H]1[C@H](O)[C@H](O)CO)[13C](O)=O
InChI
1S/C11H19NO9/c1-4(14)12-7-5(15)2-11(20,10(18)19)21-9(7)8(17)6(16)3-13/h5-9,13,15-17,20H,2-3H2,1H3,(H,12,14)(H,18,19)/t5-,6+,7+,8+,9+,11?/m0/s1/i2+1,10+1,11+1
InChI key
SQVRNKJHWKZAKO-JQVNYIDJSA-N
General description
N-Acetyl-D-neuraminic acid-1,2,3-13C3 is an isotope analog of N-Acetyl-D-neuraminic acid.
Application
N-Acetyl-D-neuraminic acid-1,2,3-13C3 can be used to quantify acyclic keto, keto hydrate, and enol forms by 13C NMR spectroscopy. It is also used to determine sialic acid supplementation as a therapeutic avenue for NANS-deficient patients.
Packaging
This product may be available from bulk stock and can be packaged on demand. For information on pricing, availability and packaging, please contact Stable Isotopes Customer Service.
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Lilian Dubois et al.
Journal of medicinal chemistry, 63(15), 8231-8249 (2020-07-02)
Sialin, encoded by the SLC17A5 gene, is a lysosomal sialic acid transporter defective in Salla disease, a rare inherited leukodystrophy. It also enables metabolic incorporation of exogenous sialic acids, leading to autoantibodies against N-glycolylneuraminic acid in humans. Here, we identified
Mark D Jankowski et al.
Comparative biochemistry and physiology. Part B, Biochemistry & molecular biology, 238, 110336-110336 (2019-09-03)
Understanding variation in physiological traits across taxa is a central question in evolutionary biology that has wide-ranging implications in biomedicine, disease ecology, and environmental protection. Sialic acid (Sia), and in particular, 5-N-acetylneuraminic acid (Neu5Ac), is chemically bound to galactose and
Christel Tran et al.
Molecular genetics and metabolism reports, 28, 100777-100777 (2021-07-15)
In NANS deficiency, biallelic mutations in the N-acetylneuraminic acid synthase (NANS) gene impair the endogenous synthesis of sialic acid (N-acetylneuraminic acid) leading to accumulation of the precursor, N-acetyl mannosamine (ManNAc), and to a multisystemic disorder with intellectual disability. The aim
Thomas Klepach et al.
Journal of the American Chemical Society, 130(36), 11892-11900 (2008-08-20)
Aqueous solutions of N-acetyl-neuraminic acid (Neu5Ac, 1) labeled with (13)C at C1, C2, and/or C3 were analyzed by (13)C NMR spectroscopy to detect and quantify the acyclic forms (keto, keto hydrate, enol) present at varying pHs. In addition to pyranoses
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service