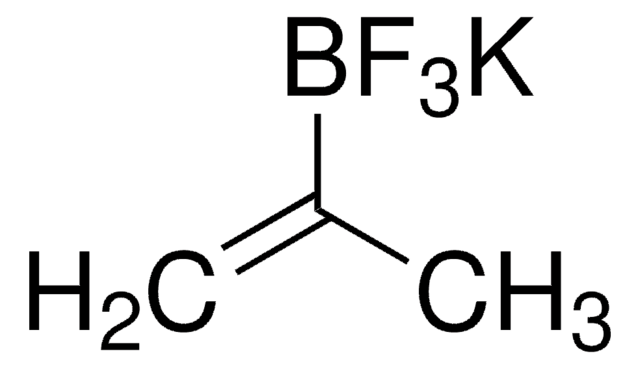
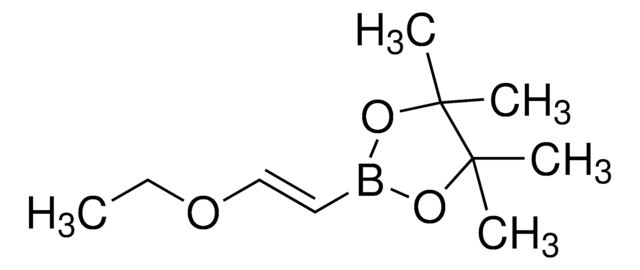
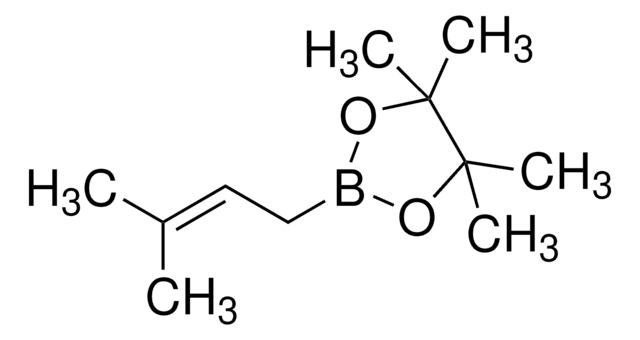
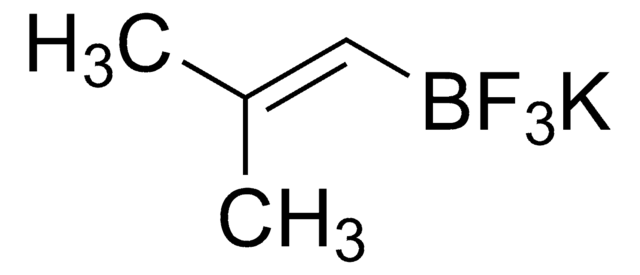
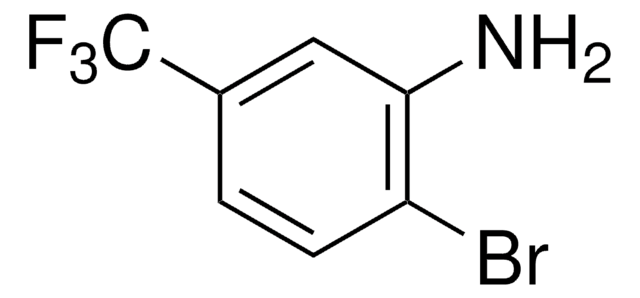
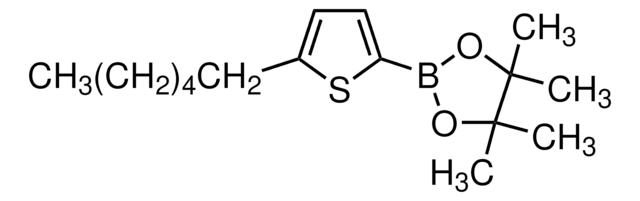
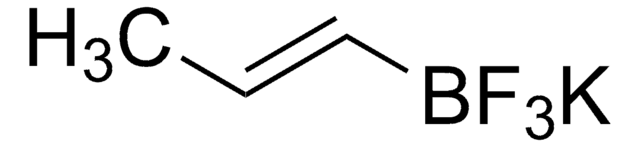
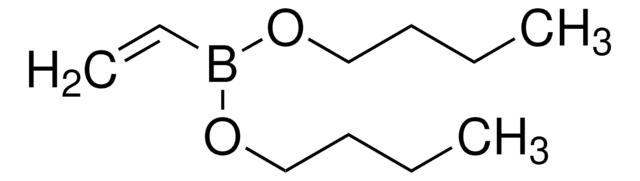
663212
Isopropenylboronic acid pinacol ester
contains phenothiazine as stabilizer, 95%
Synonym(s):
2-Isopropenyl-4,4,5,5-tetramethyl-1,3,2-dioxaborolane, 4,4,5,5-Tetramethyl-2-(isopropenyl)-1,3,2-dioxaborolane, 4,4,5,5-Tetramethyl-2-(prop-1-en-2-yl)-1,3,2-dioxaborolane, 4,4,5,5-tetramethyl-2-(1-methylethenyl)-1,3,2-dioxaborolane
About This Item
Recommended Products
Quality Level
assay
95%
contains
phenothiazine as stabilizer
refractive index
n20/D 1.4320
bp
47-49 °C/9 mbar
density
0.894 g/mL at 25 °C
storage temp.
2-8°C
SMILES string
CC(=C)B1OC(C)(C)C(C)(C)O1
InChI
1S/C9H17BO2/c1-7(2)10-11-8(3,4)9(5,6)12-10/h1H2,2-6H3
InChI key
SVSUYEJKNSMKKW-UHFFFAOYSA-N
Related Categories
Application
- Palladium-catalyzed Suzuki-Miyaura cross-coupling processes
- Inverse-electron-demand Diels-Alder reaction
- Simmons-Smith Cyclopropanation Reaction
- Polyene cyclization
- Stereoselective aldol reactions
- Grubbs cross-metathesis reaction
- Intramolecular Suzuki-Miyaura reaction
- Stereoselective cross-metathesis
- Dipolar cycloaddition
- Iodosulfonylation
- Asymmetric conjugate addition and intramolecular hydroacylation
Reagent used in preparation of various therapeutic kinase and enzymatic inhibitors
- Palladium-catalyzed Suzuki-Miyaura cross-coupling reactions
- Inverse-electron-demand Diels-Alder reaction
- Simmons-Smith Cyclopropanation Reaction
- Polyene cyclization
- Stereoselective aldol reactions
- Grubbs cross-metathesis reaction
- Intramolecular Suzuki-Miyaura reaction
- Stereoselective cross-metathesis
- Dipolar cycloaddition
- Iodosulfonylation
- Asymmetric conjugate addition and intramolecular hydroacylation
Reagent used in preparation of various therapeutic kinase and enzymatic inhibitors
signalword
Warning
Hazard Classifications
Aquatic Chronic 3 - Eye Irrit. 2 - Flam. Liq. 3 - Skin Irrit. 2 - Skin Sens. 1 - STOT SE 3
target_organs
Respiratory system
Storage Class
3 - Flammable liquids
wgk_germany
WGK 3
flash_point_f
107.6 °F
flash_point_c
42 °C
ppe
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service

![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)