670197
(S)-2-(Methoxydiphenylmethyl)pyrrolidine
95% (HPLC)
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C18H21NO
CAS Number:
Molecular Weight:
267.37
MDL number:
UNSPSC Code:
12352005
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
95% (HPLC)
optical purity
ee: ≥99.5% (HPLC)
functional group
ether
phenyl
storage temp.
2-8°C
SMILES string
COC([C@@H]1CCCN1)(c2ccccc2)c3ccccc3
InChI
1S/C18H21NO/c1-20-18(17-13-8-14-19-17,15-9-4-2-5-10-15)16-11-6-3-7-12-16/h2-7,9-12,17,19H,8,13-14H2,1H3/t17-/m0/s1
InChI key
CGUGCZSRPDCLBT-KRWDZBQOSA-N
Related Categories
Application
(S)-2-(Methoxydiphenylmethyl)pyrrolidine is a diphenylprolinol methyl ether, which can be used as a catalyst to synthesize:
- Enantioselective ketones via intermolecular asymmetric Michael addition of aldehydes to nonactivated enones.
- Stereoselective chiral bipyrazolidin-3-one derivatives by dipolar cycloaddition reaction of azomethine imines with α, β-unsaturated aldehydes.
- Enantioenriched spiro nitrogen heterocycles via asymmetric nucleophilic epoxidation of α-ylideneoxindole esters.
- Optically active secondary alcohols by asymmetric addition of Et2Zn to various aldehydes.
Packaging
Bottomless glass bottle. Contents are inside inserted fused cone.
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Diphenylprolinol methyl ether: a highly enantioselective catalyst for Michael addition of aldehydes to simple enones
Chi Y and Gellman SH
Organic Letters, 7(19), 4253-4256 (2005)
Noncovalent organocatalysis: A powerful tool for the nucleophilic epoxidation of α-ylideneoxindoles
Palumbo C, et al.
Organic Letters, 13(23), 6248-6251 (2011)
Chiral pyrrolidine derivatives as catalysts in the enantioselective addition of diethylzinc to aldehydes
Yang X, et al.
Tetrahedron Asymmetry, 10(1), 133-138 (1999)
Organocatalytic and Stereoselective [3+ 2] Cycloadditions of Azomethine Imines with , α, β-Unsaturated Aldehydes
Chen W, et al.
Advanced Synthesis & Catalysis, 348(14), 1818-1822 (2006)
Organocatalytic and Stereoselective [3+ 2] Cycloadditions of Azomethine Imines with , α, β-Unsaturated Aldehydes
Chen W, et al.
advanced synthesis and catalysis, 348(14), 1818-1822 (2006)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service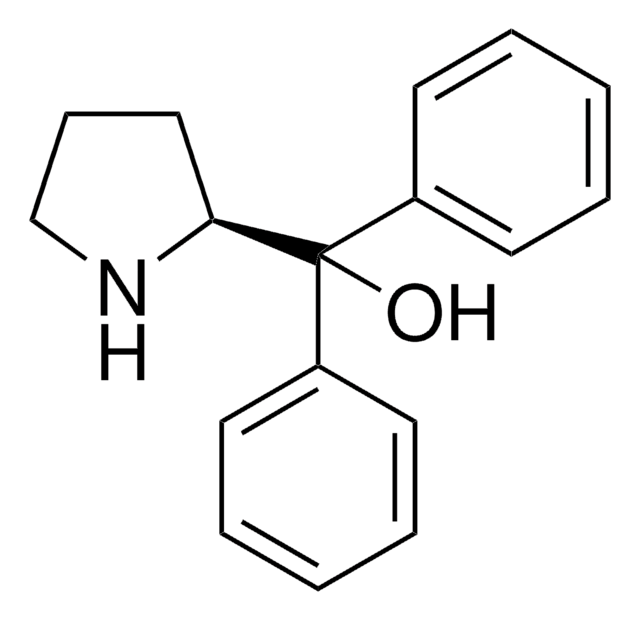
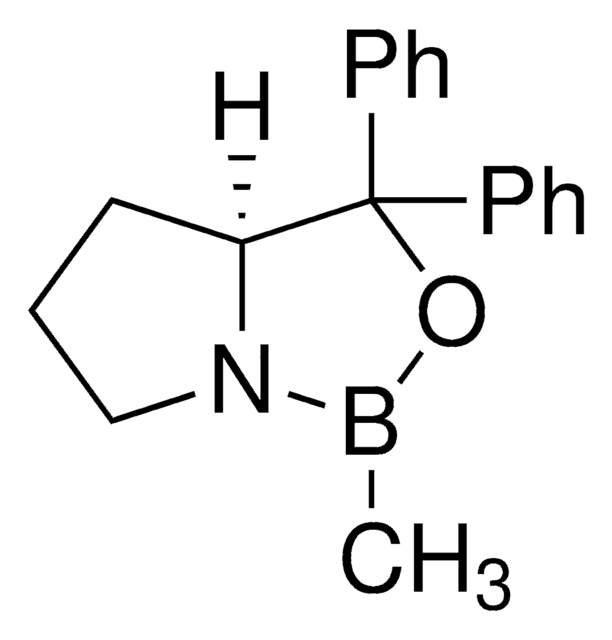
![(S)-α,α-Bis[3,5-bis(trifluoromethyl)phenyl]-2-pyrrolidinemethanol trimethylsilyl ether 97%](/deepweb/assets/sigmaaldrich/product/structures/396/398/09a397b1-b5f5-420f-98da-adf9017cef56/640/09a397b1-b5f5-420f-98da-adf9017cef56.png)
![(S)-(+)-N-(3,5-Dioxa-4-phosphacyclohepta[2,1-a;3,4-a′]dinaphthalen-4-yl)-dibenzo[b,f]azepine ≥95% (elemental analysis)](/deepweb/assets/sigmaaldrich/product/structures/575/489/d54360f9-5a59-43f2-bc44-42f5fa92b588/640/d54360f9-5a59-43f2-bc44-42f5fa92b588.png)
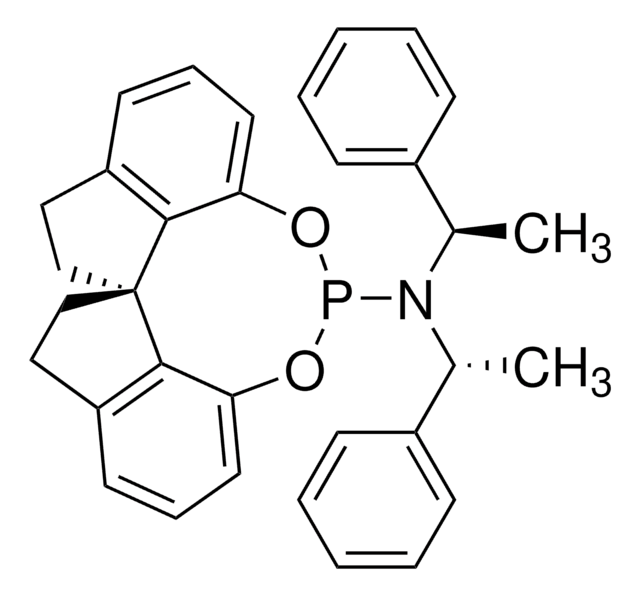
![(S,S,S)-(+)-(3,5-Dioxa-4-phosphacyclohepta[2,1-a:3,4-a’]dinaphthalen-4-yl)bis(1-phenylethyl)amine 97%](/deepweb/assets/sigmaaldrich/product/structures/223/794/16c37a96-da16-488a-b3e8-7d89c47f71ee/640/16c37a96-da16-488a-b3e8-7d89c47f71ee.png)
![(+)-Bis[(R)-1-phenylethyl]amine 99%](/deepweb/assets/sigmaaldrich/product/structures/188/828/177cd49c-056f-47d3-976c-c8cdcd5f62c5/640/177cd49c-056f-47d3-976c-c8cdcd5f62c5.png)