693057
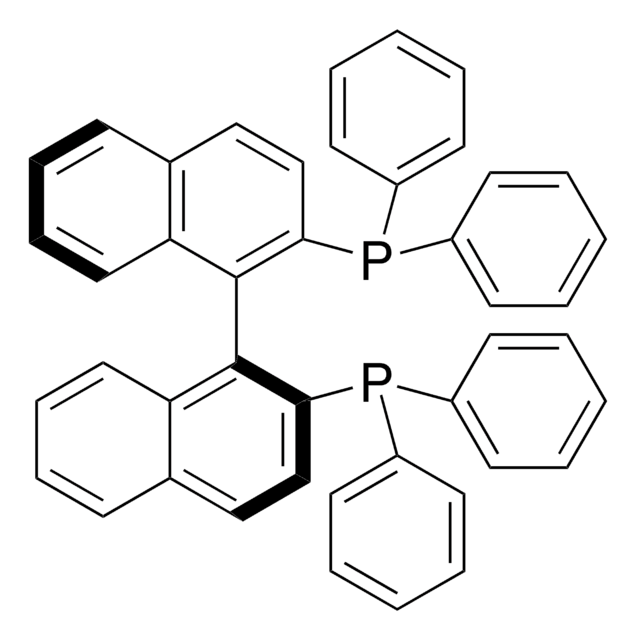
(S)-BINAP
Synonym(s):
(S)-(−)-2,2′-Bis(diphenylphosphino)-1,1′-binaphthalene, (S)-(−)-2,2′-Bis(diphenylphosphino)-1,1′-binaphthyl, (S)-(−)-(1,1′-Binaphthalene-2,2′-diyl)bis(diphenylphosphine), (S)-(−)-BINAP
About This Item
Recommended Products
form
crystals
Quality Level
optical activity
[α]20/D -222, c = 0.5 in benzene
mp
238-240 °C (lit.)
functional group
phosphine
InChI
1S/C44H32P2/c1-5-19-35(20-6-1)45(36-21-7-2-8-22-36)41-31-29-33-17-13-15-27-39(33)43(41)44-40-28-16-14-18-34(40)30-32-42(44)46(37-23-9-3-10-24-37)38-25-11-4-12-26-38/h1-32H
InChI key
MUALRAIOVNYAIW-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
- Enantioselective and diastereoselective unpoled carbonyl allylation
- Syntehsis of organophophine oxides as anittumor agents
- SN2 halogenation of hydroxy groups
- Synthesis of BINAP complexes
- Studies of conformational flexibility of BINAP chelates
Legal Information
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
We present an article concerning BINAP/SEGPHOS® Ligands and Complexes.
Hydrogenation, Asymmetric Catalysis, Binap, SEGPHOS®, Aldol reaction, Alkenylation, Arylation, Mannich reaction, Fluorination, Michael addition, Hydrosilylation, Cycloaddition, Takasago
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service