712191
COMU®
≥96.5% (TLC), for peptide synthesis
Synonym(s):
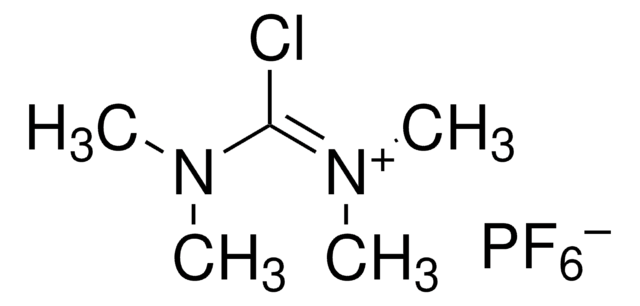
(1-Cyano-2-ethoxy-2-oxoethylidenaminooxy)dimethylamino-morpholino-carbenium hexafluorophosphate
About This Item
Recommended Products
product name
COMU®, 97%
Quality Level
assay
≥96.5% (TLC)
96.5-103.5% (T)
97%
form
crystals
reaction suitability
reaction type: Coupling Reactions
greener alternative product characteristics
Less Hazardous Chemical Syntheses
Inherently Safer Chemistry for Accident Prevention
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
application(s)
peptide synthesis
functional group
amine
ester
ether
nitrile
greener alternative category
, Aligned
storage temp.
2-8°C
SMILES string
F[P-](F)(F)(F)(F)F.CCOC(=O)C(=NO\C(N1CCOCC1)=[N+](/C)C)C#N
InChI
1S/C12H19N4O4.F6P/c1-4-19-11(17)10(9-13)14-20-12(15(2)3)16-5-7-18-8-6-16;1-7(2,3,4,5)6/h4-8H2,1-3H3;/q+1;-1/b14-10-;
InChI key
GPDHNZNLPKYHCN-DZOOLQPHSA-N
General description
Application
Advantages
- Equal or even superior performance to HATU
- Non-explosive (does not contain benzotriazole moiety)
- Suitable for solution phase & solid phase peptide synthesis
- Utmost retention of configuration – low to non-existent racemization
- High solubility and stability in typical solvents
- Visual or colorimetric reaction monitoring possible
- Easy removal of water-soluble by-products
Legal Information
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service![COMU 1-[(1-(Cyano-2-ethoxy-2-oxoethylideneaminooxy) dimethylaminomorpholino)] uronium hexafluorophosphate Novabiochem®](/deepweb/assets/sigmaaldrich/product/images/237/337/13566c06-8931-4cc2-8621-c8742a392cd6/640/13566c06-8931-4cc2-8621-c8742a392cd6.jpg)