726621
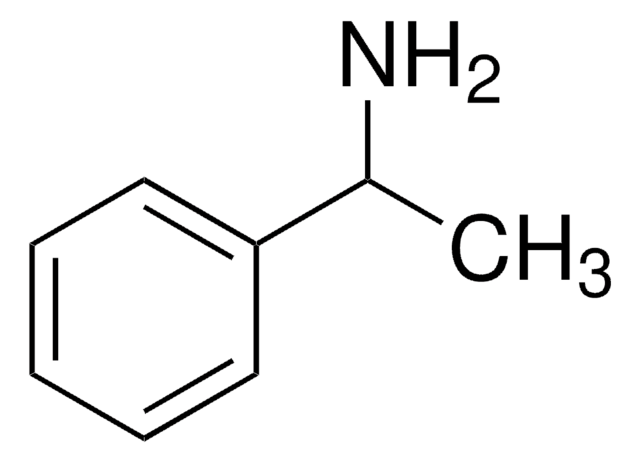
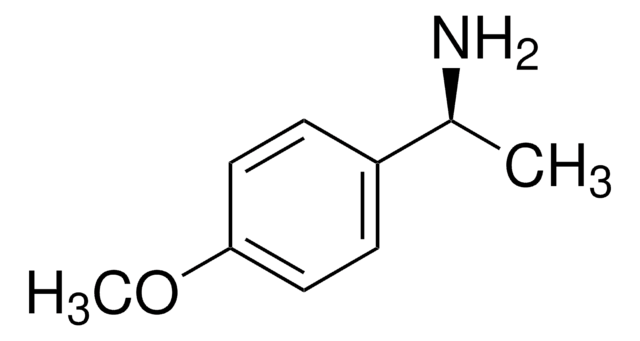
(R)-(+)-α-Methylbenzylamine
ChiPros®, produced by BASF, ≥99.0%
Synonym(s):
(R)-(+)-1-Phenylethylamine
About This Item
Recommended Products
grade
produced by BASF
Quality Level
vapor pressure
0.5 mmHg ( 20 °C)
assay
≥99.0% (GC)
≥99.0%
form
liquid
optical purity
enantiomeric excess: ≥98.0%
refractive index
n20/D 1.526 (lit.)
bp
187-189 °C (lit.)
density
0.952 g/mL at 20 °C (lit.)
functional group
amine
phenyl
SMILES string
C[C@@H](N)c1ccccc1
InChI
1S/C8H11N/c1-7(9)8-5-3-2-4-6-8/h2-7H,9H2,1H3/t7-/m1/s1
InChI key
RQEUFEKYXDPUSK-SSDOTTSWSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
- (S)-α-amino phosphonates
- N-{(S)-[cyclohexan-(S)-2-ol]}-(R)-α-methylbenzyl amine and N-{(R)-[cyclohexan-(R)-2-ol]}-(R)-α-methylbenzyl amine
- R-α-aminonitriles
Legal Information
signalword
Danger
hcodes
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Oral - Eye Dam. 1 - Skin Corr. 1B
Storage Class
8A - Combustible corrosive hazardous materials
wgk_germany
WGK 1
flash_point_f
158.0 °F - closed cup
flash_point_c
70 °C - closed cup
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Chiral amines play an important role in stereoselective organic synthesis. They are used directly as resolving agents, building blocks, or chiral auxiliaries.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service