727989
Ethyldimethylpropylammonium bis(trifluoromethylsulfonyl)imide
99%
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C9H18F6N2O4S2
CAS Number:
Molecular Weight:
396.37
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
99%
form
liquid
impurities
≤1.0% water
functional group
amine
fluoro
SMILES string
CCC[N+](C)(C)CC.FC(F)(F)S(=O)(=O)[N-]S(=O)(=O)C(F)(F)F
InChI
1S/C7H18N.C2F6NO4S2/c1-5-7-8(3,4)6-2;3-1(4,5)14(10,11)9-15(12,13)2(6,7)8/h5-7H2,1-4H3;/q+1;-1
InChI key
FKXJTTMLNYZAOH-UHFFFAOYSA-N
General description
Ethyldimethylpropylammonium bis(trifluoromethylsulfonyl)imide is an ionic liquid with high dielectrical permittivity, ionic conductivity and low viscosity.
Storage Class
10 - Combustible liquids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Confinement of 1-butyl-3-methylimidazolium nitrate in metallic silver
Neouze, Marie-Alexandra, and Marco Litschauer
The Journal of Physical Chemistry, 112.51, 16721-16725 (2008)
Parametrization of 1-butyl-3-methylimidazolium hexafluorophosphate/nitrate ionic liquid for the GROMOS force field
Micaelo, Nuno M., Antonio M. Baptista, and Claudio M. Soares
Analytical Chemistry, 110.29, 14444-14451 (2006)
Thermochemical properties of 1-butyl-3-methylimidazolium nitrate
Strechan, A. A., et al.
Electrochimica Acta, 474.1, 25-31 (2008)
Electrical double layer capacitors with sucrose derived carbon electrodes in ionic liquid electrolytes.
Wei L and Yushin G.
Journal of Power Sources, 196(8), 4072-4079 (2011)
Abderrahman Atifi et al.
Molecules (Basel, Switzerland), 25(2) (2020-01-17)
Understanding the solvation and ion-pairing interactions of anionic substrates in room-temperature ionic liquids (RTIL) is key for the electrochemical applications of these new classes of solvents. In this work, cyclic voltammetry and visible and infrared spectroelectrochemistry of tetracyanoquinodimethane (TCNQ) was
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service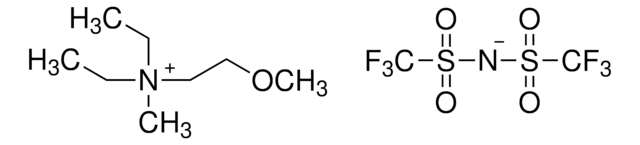




![Zinc di[bis(trifluoromethylsulfonyl)imide] 95%](/deepweb/assets/sigmaaldrich/product/structures/336/073/952daadd-0a7c-4bec-bbaf-442a24c62161/640/952daadd-0a7c-4bec-bbaf-442a24c62161.png)


