76172
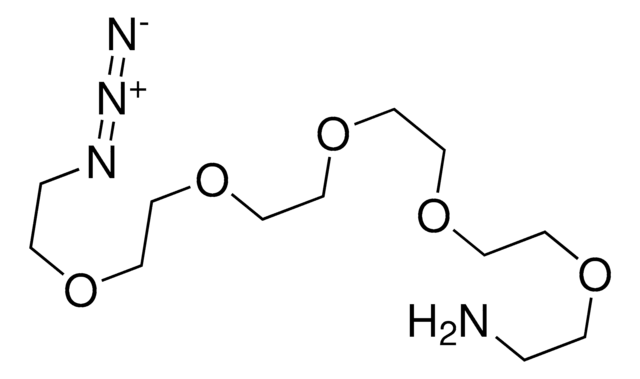
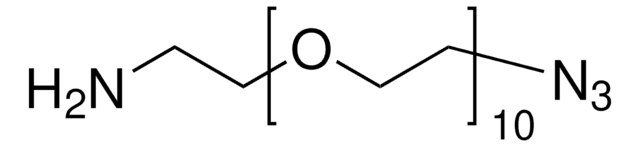
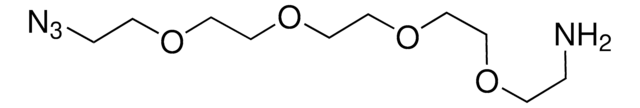
O-(2-Aminoethyl)-O′-(2-azidoethyl)pentaethylene glycol
≥90% (oligomer purity)
Synonym(s):
Azido-PEG-amine (n=6)
About This Item
Recommended Products
Quality Level
assay
≥90% (oligomer purity)
form
liquid
reaction suitability
reaction type: click chemistry
reagent type: cross-linking reagent
functional group
amine
azide
storage temp.
2-8°C
SMILES string
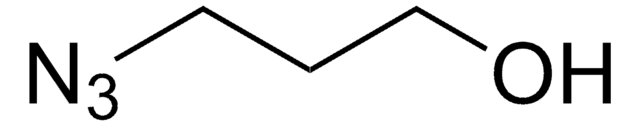
NCCOCCOCCOCCOCCOCCOCCN=[N+]=[N-]
InChI
1S/C14H30N4O6/c15-1-3-19-5-7-21-9-11-23-13-14-24-12-10-22-8-6-20-4-2-17-18-16/h1-15H2
InChI key
VCQSTKKJKNUQBI-UHFFFAOYSA-N
Application
- As a reactant for the synthesis of thin films of azide functionalized poly(L-glutamic acid) for drug delivery.[1]
- For the synthesis of curcumin monoazide derivatives for fabricating biologically active curcumin conjugates.[2]
- To modify the surface of TiO2 nanoparticles to attach DNA strands for photocatalytic reduction of CO2.[3]
- For the conjugation of poly(ethylene glycol) (PEG) with toll-like receptor 7 (TLR7) to improve its pharmaceutical properties.[4]
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
10 - Combustible liquids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service




![2-[2-(2-Azidoethoxy)ethoxy]ethanol solution ~0.5 M in tert-butyl methyl ether](/deepweb/assets/sigmaaldrich/product/structures/374/007/eea7ca74-41e4-4aac-af71-c93c37ec0a5a/640/eea7ca74-41e4-4aac-af71-c93c37ec0a5a.png)