777161
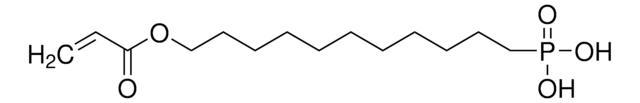
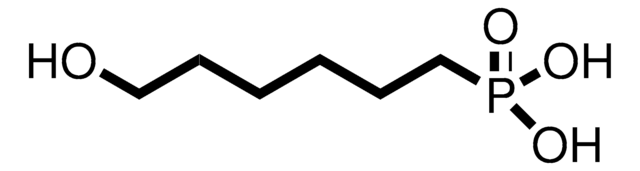
11-Hydroxyundecylphosphonic acid
≥95% (GC)
Synonym(s):
P-(11-Hydroxyundecyl)phosphonic acid
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C11H25O4P
CAS Number:
Molecular Weight:
252.29
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
Quality Level
assay
≥95% (GC)
form
powder
mp
107-111 °C
SMILES string
OCCCCCCCCCCCP(O)(O)=O
InChI
1S/C11H25O4P/c12-10-8-6-4-2-1-3-5-7-9-11-16(13,14)15/h12H,1-11H2,(H2,13,14,15)
InChI key
PPCDEFQVKBXBPS-UHFFFAOYSA-N
General description
11-Hydroxyundecylphosphonic acid (HUPA) is an alkyl phosphonic acid that forms a self-assembled monolayer (SAM) on a variety of metal oxides and metal surfaces. It forms a hydroxyl terminated SAM that provides a physiological stability and controls the surface density of the coating.
Application
An organophosphonate surface modifier for label-free and low-cost biosensing applications;
Organophosphonic acid self-assembled monolayers (SAMs);
A hydroxyalkylphosphonate monolayer used to covalently bind primary amine groups in protein domains using chloroformate-derived crosslinking
Organophosphonic acid self-assembled monolayers (SAMs);
A hydroxyalkylphosphonate monolayer used to covalently bind primary amine groups in protein domains using chloroformate-derived crosslinking
HUPA is mainly used as a spacer/linker that forms a SAM on titanium oxide (TiO2) surfaces to immobilize the surface atoms for the formation of biocompatible materials for biomedical applications. It can also be used to functionalize the silicon-photonic resonators for biosensing applications.
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Crystalline TiO2 grafted with poly (2-methacryloyloxyethyl phosphorylcholine) via surface-initiated atom-transfer radical polymerization
Zhao Y, et al.
Applied Surface Science, 257(5), 1596-1601 (2010)
Covalent functionalization of TiO2 nanotube arrays with EGF and BMP-2 for modified behavior towards mesenchymal stem cells
Bauer S, et al.
Integrative Biology : Quantitative Biosciences from Nano to Macro, 3(9), 927-936 (2011)
Copper oxide surfaces modified by alkylphosphonic acids with terminal pyridyl-based ligands as a platform for supported catalysis
Andrews B, et al.
Polyhedron, 114, 360-369 (2016)
Chunyi Chiang et al.
Langmuir : the ACS journal of surfaces and colloids, 28(1), 548-556 (2011-11-23)
We report a robust strategy for conjugating mixtures of two or more protein domains to nonfouling polyurethane surfaces. In our strategy, the carbamate groups of polyurethane are reacted with zirconium alkoxide from the vapor phase to give a surface-bound oxide
Phosphonic acid monolayers for binding of bioactive molecules to titanium surfaces.
Adden N
Langmuir, 22(19), 8197-8204 (2006)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service