86734
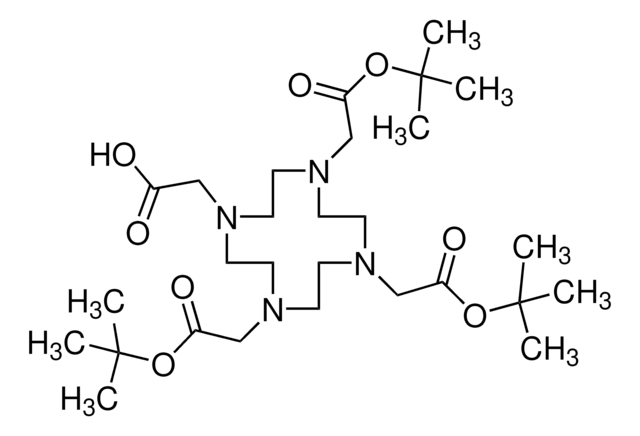
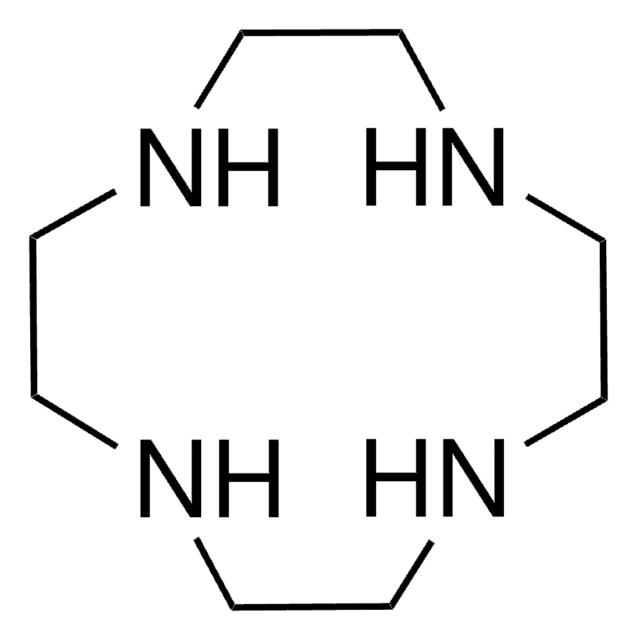
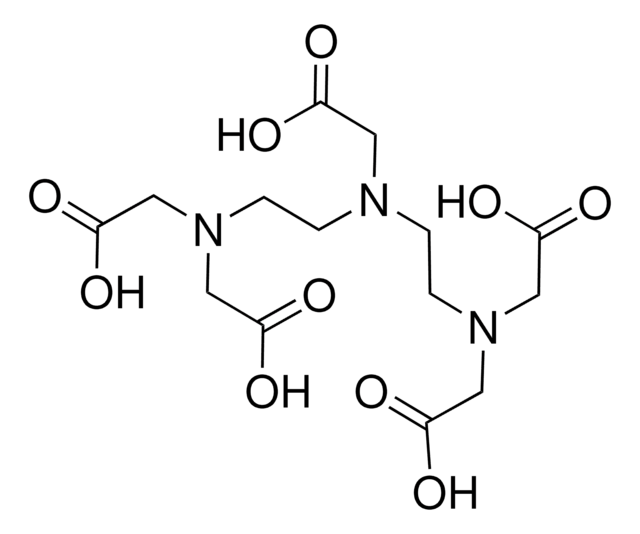
1,4,7,10-Tetraazacyclododecane-1,4,7,10-tetraacetic acid
≥97.0% (CHN)
Synonym(s):
DOTA
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C16H28N4O8 · xH2O
CAS Number:
Molecular Weight:
404.42 (anhydrous basis)
Beilstein/REAXYS Number:
1186987
MDL number:
UNSPSC Code:
12352005
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
≥97.0% (CHN)
form
powder
functional group
carboxylic acid
SMILES string
OC(=O)CN1CCN(CCN(CCN(CC1)CC(O)=O)CC(O)=O)CC(O)=O
InChI
1S/C16H28N4O8/c21-13(22)9-17-1-2-18(10-14(23)24)5-6-20(12-16(27)28)8-7-19(4-3-17)11-15(25)26/h1-12H2,(H,21,22)(H,23,24)(H,25,26)(H,27,28)
InChI key
WDLRUFUQRNWCPK-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
1,4,7,10-Tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA) is a macrocyclic complexing agent.
- It has been used for radiolabeling of carbon nanotube bioconjugates by chelating 64Cu radioisotope.
- DOTA can be activated with N-hydroxysulfosuccinimidyl for conjugation with monoclonal antibodies in clinical radioimmunotherapy.
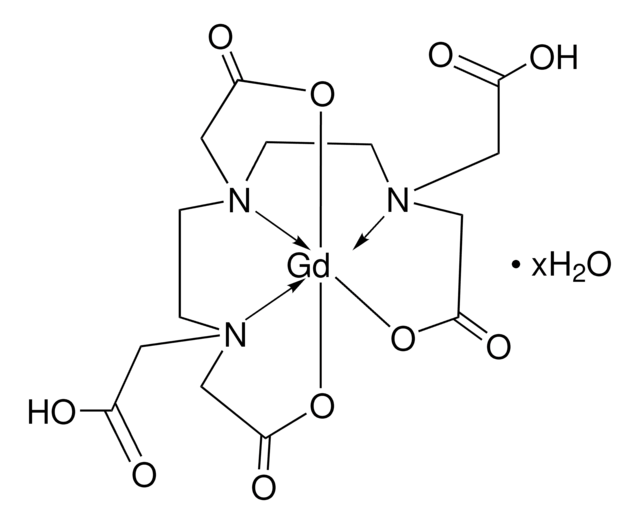
- It can form complexes with gadolinium for application as MRI contrast agents.
- Metal complexes of DOTA-peptide conjugates can be used as therapeutic radiopharmaceuticals.
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
An improved method for conjugating monoclonal antibodies with N-hydroxysulfosuccinimidyl DOTA.
Lewis MR, et al.
Bioconjugate Chemistry, 12(2), 320-324 (2001)
The synthesis and chelation chemistry of DOTA- peptide conjugates.
De Leon-Rodriguez LM and Kovacs Z
Bioconjugate Chemistry, 19(2), 391-402 (2007)
Javad Garousi et al.
Scientific reports, 7(1), 14780-14780 (2017-11-09)
ABD-Derived Affinity Proteins (ADAPTs) is a novel class of engineered scaffold proteins derived from an albumin-binding domain of protein G. The use of ADAPT6 derivatives as targeting moiety have provided excellent preclinical radionuclide imaging of human epidermal growth factor 2
In vivo biodistribution and highly efficient tumour targeting of carbon nanotubes in mice.
Liu Z, et al.
Nature Nanotechnology, 2(1), 47-47 (2007)
GdIII complexes with fast water exchange and high thermodynamic stability: potential building blocks for high-relaxivity MRI contrast agents.
Laus S, et al.
Chemistry?A European Journal , 9(15), 3555-3566 (2003)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service