900878
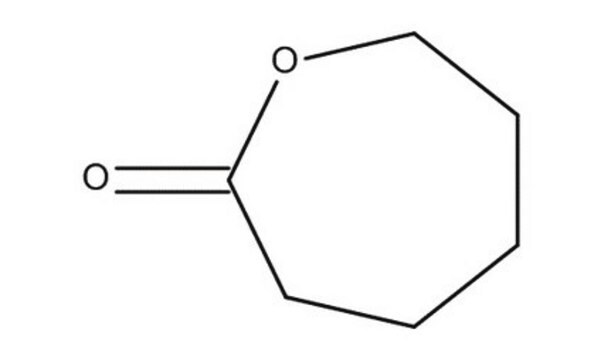
2-Chloro-ε-caprolactone
Synonym(s):
α-Chloro-ε-caprolactone, αClεCL, 2-Chloro-1-oxacycloheptan-2-one
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C6H9ClO2
CAS Number:
Molecular Weight:
148.59
MDL number:
UNSPSC Code:
12352101
NACRES:
NA.23
Recommended Products
assay
98% (NMR)
Quality Level
form
liquid
color
colorless to faint yellow
storage temp.
−20°C
SMILES string
O=C1C(Cl)CCCCO1
General description
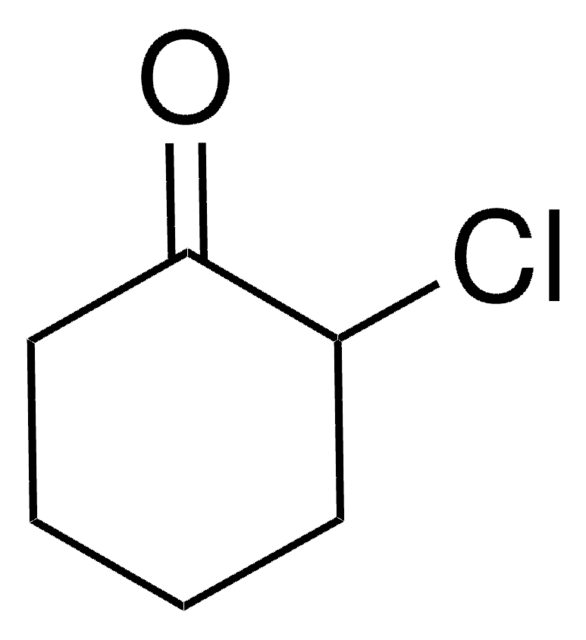
2-Chloro-ε-caprolactone (ClCL) is a biomaterial that can be prepared by the Baeyer-Villiger oxidation of α-chlorocyclohexanone. It can be copolymerized with ε-caprolactone for the formation of Poly(2-chloro-ε-caprolactone).
Application
2-Chloro-ε-caprolactone (or α-Chloro-ε-caprolactone) is a functionalized biodegradable monomer. This monomer can be polymerized using ring-opening polymerization to yield a chloride-functionalized polymer backbone that can either be further functionalized with small molecules or used in the synthesis of graft co-polymers.
Storage Class
10 - Combustible liquids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Development of Core?Shell Nanostructures by In Situ Assembly of Pyridine-Grafted Diblock Copolymer and Transferrin for Drug Delivery Applications
Lu L , et al.
Biomacromolecules, 17 (7), 2321-2328 (2016)
Ring-Opening Polymerization of α-Chloro-ε-caprolactone and Chemical Modification of Poly(α-chloro-ε-caprolactone
Lenoir S. et al
Macromolecules, 37 (11), 4055-4061 (2004)
Aniline-Catalyzed Reductive Amination as a Powerful Method for the Preparation of Reducing End-?Clickable? Chitooligosaccharides
Guerry A, et al.
Bioconjugate Chemistry, 24 (4), 544?9 -544?9 (2013)
Synthesis of Poly(lactide-co-glycolide-co-ε-caprolactone)-graft-mannosylated Poly(ethylene oxide) Copolymers by Combination of ?Clip? and ?Click? Chemistries
Biomacromolecules, 13 (3), 760-78 (2012)
Ring-opening polymerization of alpha-chloro-varepsilon-caprolactone and chemical modification of poly (alpha-chloro-varepsilon-caprolactone) by atom transfer radical processes
Lenoir S, et al.
Macromolecules, 37(11), 4055-4061 (2004)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service