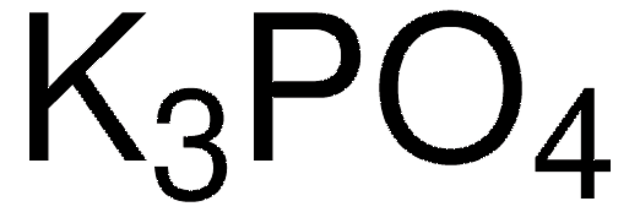901906
SPhos
95%
Synonym(s):
2-Dicyclohexylphosphino-2′,6′-dimethoxybiphenyl, S Phos
About This Item
Recommended Products
assay
95%
form
powder or crystals
reaction suitability
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reaction type: Heck Reaction
reaction type: Hiyama Coupling
reaction type: Negishi Coupling
reaction type: Sonogashira Coupling
reaction type: Stille Coupling
reaction type: Suzuki-Miyaura Coupling
reagent type: catalyst
reaction type: Cross Couplings
reagent type: ligand
mp
164-166 °C (lit.)
165.5 °C
functional group
phosphine
SMILES string
COc1cccc(OC)c1-c2ccccc2P(C3CCCCC3)C4CCCCC4
InChI
1S/C26H35O2P/c1-27-23-17-11-18-24(28-2)26(23)22-16-9-10-19-25(22)29(20-12-5-3-6-13-20)21-14-7-4-8-15-21/h9-11,16-21H,3-8,12-15H2,1-2H3
InChI key
VNFWTIYUKDMAOP-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
- Palladium catalyzed Suzuki-Miyaura cross-coupling reaction between Boc-protected aminomethyltrifluoroborate and aryl chlorides or hetaryl chlorides to form the corresponding aminomethylarenes.
- Palladium catalyzed Suzuki-Miyaura cross-coupling reaction between 4-methyl-substituted piperidinylzinc reagent and different aryl or heteroaryl iodides to form various substituted piperidines.
- Intramolecular Suzuki-Miyaura coupling to form the 18-membered macrocyclic ring during the multi-step synthesis of riccardin C.
Legal Information
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service


![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)