A38207
2-Aminoacetophenone hydrochloride
99%
Synonym(s):
ω-Aminoacetophenone hydrochloride, Phenacylamine hydrochloride
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
C6H5COCH2NH2 · HCl
CAS Number:
Molecular Weight:
171.62
Beilstein/REAXYS Number:
3563173
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
99%
form
crystals
mp
194 °C (dec.) (lit.)
SMILES string
Cl.NCC(=O)c1ccccc1
InChI
1S/C8H9NO.ClH/c9-6-8(10)7-4-2-1-3-5-7;/h1-5H,6,9H2;1H
InChI key
CVXGFPPAIUELDV-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
[Anorexigenic activity of various derivatives of alpha-aminoacetophenone].
M S Sánchez et al.
Archivos de farmacologia y toxicologia, 5(3), 165-168 (1979-12-01)
M J Bossard et al.
The Journal of biological chemistry, 265(10), 5640-5647 (1990-04-05)
A mechanism for beta-chlorophenethylamine inhibition of dopamine beta-monooxygenase has been postulated in which bound alpha-aminoacetophenone is generated followed by an intramolecular redox reaction to yield a ketone-derived radical cation as the inhibitory species (Mangold, J.B., and Klinman, J.P. (1984) J.
Frank Sporkert et al.
Forensic science international, 133(1-2), 39-46 (2003-05-14)
A sensitive and reproducible method for the quantitative determination of cathinone (CTN), norpseudoephedrine (NPE, cathine) and norephedrine (NE) from hair was developed. The compounds were extracted for 4 hours with phosphate buffer pH 2.0, followed by a standard solid phase
Yasumasa Iwai et al.
Chemical & pharmaceutical bulletin, 50(3), 441-443 (2002-03-26)
The new coupling reaction of phenacylamines with silylstannane and lithium diisopropylamide (LDA) is reported. The treatment of a phenacylamine iodide 1 with (trimethylsilyl)tributylstannane (Me3SiSnBu3) and cesium fluoride (CsF) gave a dimerization product 2 having no iodine atom. Reaction of 1
J B Mangold et al.
The Journal of biological chemistry, 259(12), 7772-7779 (1984-06-25)
Functionalization of the beta-carbon of phenethylamines has been shown to produce a new class of substrate/inhibitor of dopamine beta-monooxygenase. Whereas both beta-hydroxy- and beta- chlorophenethylamine are converted to alpha-aminoacetophenone at comparable rates, only the latter conversion is accompanied by concomitant
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service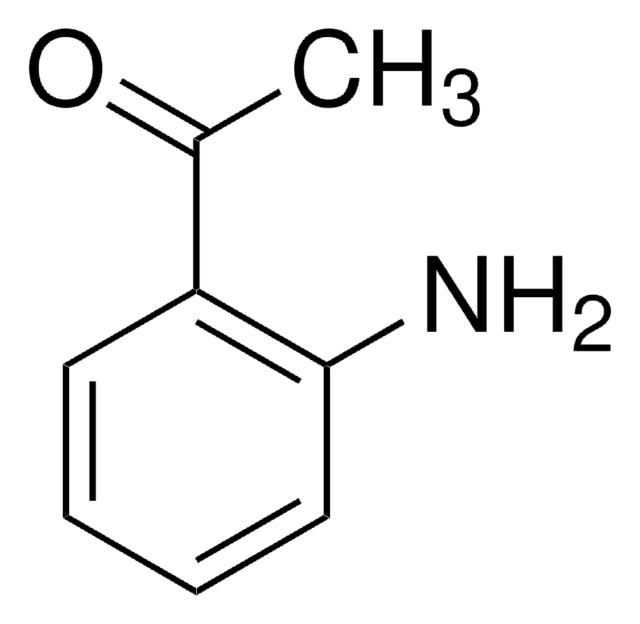

![2-[(1R)-1-Aminoethyl]-phenol AldrichCPR](/deepweb/assets/sigmaaldrich/product/structures/166/868/86b5a88f-7c6b-4634-aedc-c523a10e54aa/640/86b5a88f-7c6b-4634-aedc-c523a10e54aa.png)