All Photos(4)
About This Item
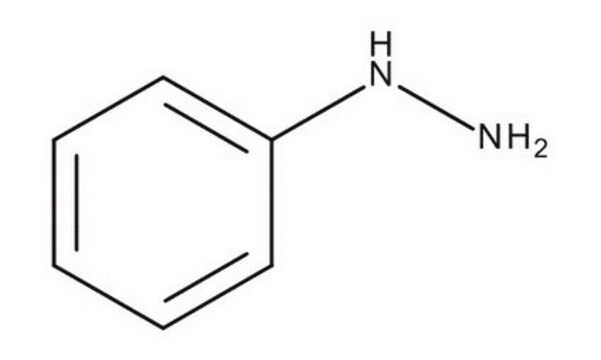
Linear Formula:
C6H5CH2NHNH2 · 2HCl
CAS Number:
Molecular Weight:
195.09
Beilstein/REAXYS Number:
3688990
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
97%
form
powder
mp
143-145 °C (dec.) (lit.)
SMILES string
Cl.Cl.NNCc1ccccc1
InChI
1S/C7H10N2.2ClH/c8-9-6-7-4-2-1-3-5-7;;/h1-5,9H,6,8H2;2*1H
InChI key
MSJHOJKVMMEMNX-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
L Morpurgo et al.
Biology of metals, 3(2), 114-117 (1990-01-01)
The role of copper in bovine serum amine oxidase was investigated by studying the effect of copper-binding inhibitors on the reactions of the pyrroloquinoline quinone carbonyl and on the reaction with oxygen. Hydrazines and hydrazides were used as carbonyl reagents
A Bellelli et al.
European journal of biochemistry, 267(11), 3264-3269 (2000-05-29)
The presteady-state and steady-state kinetics of bovine serum amine oxidase (BSAO) were analyzed by stopped-flow transient spectroscopy. A simplified model of the catalytic cycle was found to describe the experimental data and the rate constants of the individual steps were
D J Merkler et al.
Archives of biochemistry and biophysics, 317(1), 93-102 (1995-02-20)
Peptidylglycine alpha-amidating enzyme catalyzes the two-step conversion of C-terminal glycine-extended peptides to C-terminal alpha-amidated peptides and glyoxylate in a reaction that requires O2, ascorbate and 2 mol of copper per mole of enzyme [Kulathila et al. (1994) Arch. Biochem. Biophys.
David B Langley et al.
Acta crystallographica. Section F, Structural biology and crystallization communications, 64(Pt 7), 577-583 (2008-07-09)
Complexes of Arthrobacter globiformis amine oxidase (AGAO) with the inhibitors benzylhydrazine and tranylcypromine (an antidepressant drug) have been refined at 1.86 and 1.65 A resolution, respectively. Both inhibitors form covalent adducts with the TPQ cofactor. A tyrosine residue, proposed to
Han-Zhong Zhang et al.
Bioorganic & medicinal chemistry, 12(13), 3649-3655 (2004-06-10)
A series of indole-2-carboxylic acid benzylidene-hydrazides has been identified as a new class of potent apoptosis inducers through a novel cell-based caspase HTS assay. The screening hit, 5-chloro-3-methyl-indole-2-carboxylic acid (4-nitrobenzylidene)-hydrazide (3a), was found to arrest T47D cells in G(2)/M and
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service