B56501
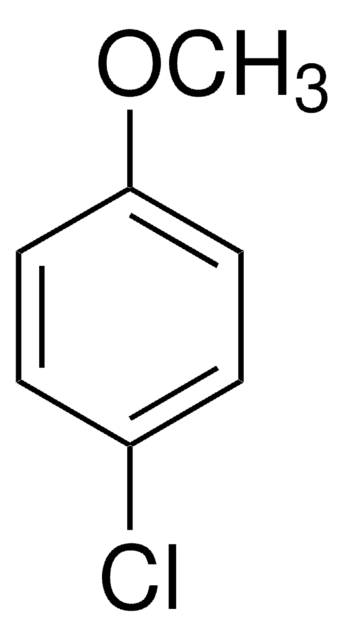
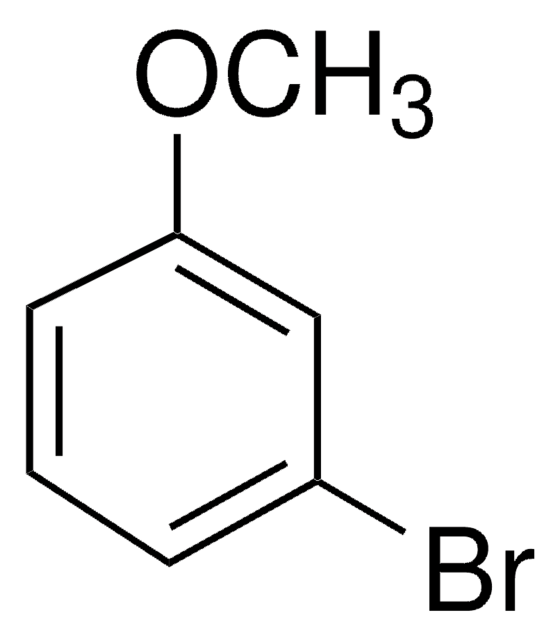
4-Bromoanisole
≥99.0%
Synonym(s):
1-Bromo-4-methoxybenzene
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
BrC6H4OCH3
CAS Number:
Molecular Weight:
187.03
Beilstein/REAXYS Number:
1237590
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
≥99.0%
form
liquid
refractive index
n20/D 1.564 (lit.)
bp
223 °C (lit.)
mp
9-10 °C (lit.)
density
1.494 g/mL at 25 °C (lit.)
SMILES string
COc1ccc(Br)cc1
InChI
1S/C7H7BrO/c1-9-7-4-2-6(8)3-5-7/h2-5H,1H3
InChI key
QJPJQTDYNZXKQF-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
4-Bromoanisole is a useful brominating reagent. It is formed as reaction product in the reaction between HOBr and anisole. Suzuki coupling of 4-bromoanisole with phenylboronic acid catalyzed by palladium pincer complexes has been studied. Heck Reaction of 4-bromoanisole with ethyl acrylates in room-temperature ionic liquids is reported to afford ethyl 4-methoxycinnamate.
Application
4-Bromoanisole was used in the synthesis of aryl 1,3-diketones.
signalword
Warning
hcodes
Hazard Classifications
Acute Tox. 4 Oral - Skin Irrit. 2
Storage Class
10 - Combustible liquids
wgk_germany
WGK 2
flash_point_f
201.2 °F
flash_point_c
94 °C
ppe
Eyeshields, Gloves, multi-purpose combination respirator cartridge (US)
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Amy E Bryant et al.
PloS one, 12(2), e0172486-e0172486 (2017-03-01)
Acute muscle injuries are exceedingly common and non-steroidal anti-inflammatory drugs (NSAIDs) are widely consumed to reduce the associated inflammation, swelling and pain that peak 1-2 days post-injury. While prophylactic use or early administration of NSAIDs has been shown to delay
Yasuyuki Nakamura et al.
Applied and environmental microbiology, 84(15) (2018-05-29)
The methylotrophic yeast Pichia pastoris is widely used to produce recombinant proteins, taking advantage of this species' high-density cell growth and strong ability to secrete proteins. Circular plasmids containing the P. pastoris-specific autonomously replicating sequence (PARS1) permit transformation of P.
J Vokurka et al.
Polish journal of veterinary sciences, 23(2), 169-176 (2020-07-07)
Different approaches to enhance healing of hard or soft tissues include the use of cytokines and growth factors to modify cellular behaviour. Numerous growth factors are found in autologous blood concentrates - platelet-rich plasma (PRP) and platelet-rich fibrin (PRF). Enamel
Valdecir Farias Ximenes et al.
Journal of inorganic biochemistry, 146, 61-68 (2015-03-17)
Hypobromous acid (HOBr) is an inorganic acid produced by the oxidation of the bromide anion (Br(-)). The blood plasma level of Br(-) is more than 1,000-fold lower than that of chloride anion (Cl(-)). Consequently, the endogenous production of HOBr is
Charles E Wood et al.
Carcinogenesis, 36(7), 782-791 (2015-04-29)
Environmental exposures occurring early in life may have an important influence on cancer risk later in life. Here, we investigated carryover effects of dichloroacetic acid (DCA), a small molecule analog of pyruvate with metabolic programming properties, on age-related incidence of
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service