B65705
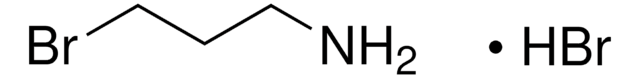
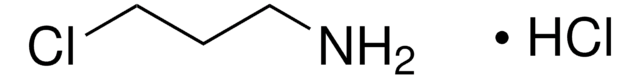
2-Bromoethylamine hydrobromide
99%
Synonym(s):
2-Aminoethyl bromide hydrobromide
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
BrCH2CH2NH2 · HBr
CAS Number:
Molecular Weight:
204.89
Beilstein/REAXYS Number:
3607605
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
99%
form
crystals
mp
170-175 °C (lit.)
SMILES string
Br.NCCBr
InChI
1S/C2H6BrN.BrH/c3-1-2-4;/h1-2,4H2;1H
InChI key
WJAXXWSZNSFVNG-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
2-Bromoethylamine hydrobromide can be used as a reactant to synthesize:
- Thiazolines and thiazines via tandem S-alkylation-cyclodeamination of thioamides/haloamines.
- Optically active tertiary phosphines via cesium hydroxide-catalyzed P-alkylation of secondary phosphines.
- Secondary amines via CsOH-promoted chemoselective mono-N-alkylation of primary amines, diamines, and polyamines.
signalword
Warning
hcodes
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Chronic 3
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
142.9 °F - Pensky-Martens closed cup
flash_point_c
61.6 °C - Pensky-Martens closed cup
ppe
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthesis, 1040-1040 (2006)
Minjoo Yeom et al.
Veterinary journal (London, England : 1997), 204(2), 157-161 (2015-04-30)
In Korea, porcine reproductive and respiratory syndrome (PRRS) is caused by European (type 1) and North American (type 2) strains of PRRS virus (PRRSV). In the present study, the efficacy of a multi-strain PRRSV vaccine inactivated with binary ethylenimine (BEI)
Marina Rodríguez et al.
BMC veterinary research, 16(1), 322-322 (2020-09-03)
African horse sickness (AHS) is a serious viral disease of equids resulting in the deaths of many equids in sub-Saharan Africa that has been recognized for centuries. This has significant economic impact on the horse industry, despite the good husbandry
G Bergström et al.
Journal of hypertension, 19(3 Pt 2), 659-665 (2001-05-01)
Restoring renal perfusion pressure (unclipping) of one-kidney-one-clip renal hypertensive (1 K1C) rats normalizes mean arterial pressure (MAP) rapidly. This has been attributed to salt/volume losses or release of the putative renal medullary depressor hormone (RMDH). To investigate the effects of
Samer Sourial et al.
Biomarkers : biochemical indicators of exposure, response, and susceptibility to chemicals, 15(5), 424-435 (2010-05-25)
Currently there are no biomarkers for detecting collecting duct damage in man. Antibodies to several collecting duct-specific antigens exist but sandwich assays have been difficult to establish due to the need for two different antibodies to the same protein. We
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service