C116807
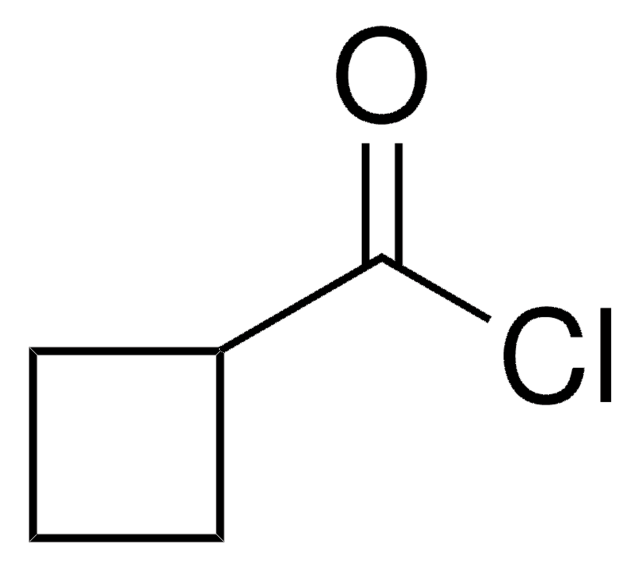
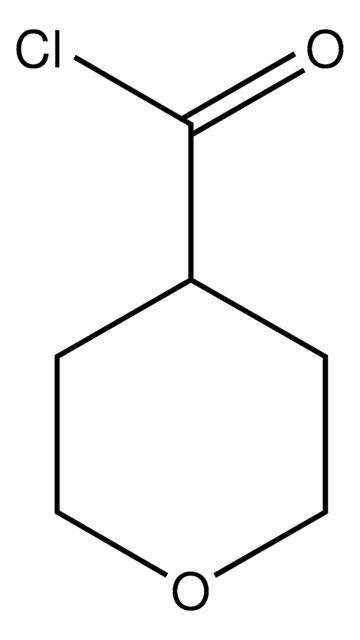
Cyclopropanecarbonyl chloride
98%
Synonym(s):
Cyclopropanecarboxylic acid chloride
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
C3H5COCl
CAS Number:
Molecular Weight:
104.53
Beilstein/REAXYS Number:
471286
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
98%
form
liquid
refractive index
n20/D 1.452 (lit.)
bp
119 °C (lit.)
density
1.152 g/mL at 25 °C (lit.)
storage temp.
2-8°C
SMILES string
ClC(=O)C1CC1
InChI
1S/C4H5ClO/c5-4(6)3-1-2-3/h3H,1-2H2
InChI key
ZOOSILUVXHVRJE-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Cyclopropanecarbonyl chloride can be used as an alkylating reagent in the preparation of some chemical building blocks such as formylcyclopropane, cyclopropanecarboxylic acid hydrazide, bis(cyclopropylcarbonyl) peroxide, 1-cyclopropanecarboxamide, 2-cyclopropylcarbonylcyclohexane-1,3-diones and cyclopropyl analog of triazolopiperazine-amides.
signalword
Danger
hcodes
Hazard Classifications
Acute Tox. 3 Oral - Eye Dam. 1 - Flam. Liq. 3 - Skin Corr. 1B
Storage Class
3 - Flammable liquids
wgk_germany
WGK 3
ppe
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Enzyme inhibition potency enhancement by active site metal chelating and hydrogen bonding induced conformation-restricted cyclopropanecarbonyl derivatives
Kuo P-Y, et al.
Bioorganic & Medicinal Chemistry Letters, 16(23), 6024-6027 (2006)
Preparation of Pivaloyl Hydrazide in Water: (Propanoic acid, 2, 2-dimethyl-, hydrazide)
Li Bryan, et al.
Organic Syntheses, 81(23), 254-261 (2003)
Palladium (II)-catalyzed highly enantioselective C-H arylation of cyclopropylmethylamines
Chan Kelvin SL, et al.
Journal of the American Chemical Society, 137(5), 2042-2046 (2015)
Triazolopiperazine-amides as dipeptidyl peptidase IV inhibitors: Close analogs of JANUVIA?(sitagliptin phosphate)
Kim, Dooseop, et al.
Bioorganic & Medicinal Chemistry Letters, 17(12), 3373-3377 (2007)
Yi Liu et al.
Journal of environmental sciences (China), 90, 180-188 (2020-02-23)
A novel N,N-dithenoyl-rhodamine based fluorescent and colorimetric Fe3+ probe 1 was designed and synthesized by only one step from Rhodamine B hydrazide and 2-thiophenecarbonyl chloride. The structure of probe 1 was characterized by 1H NMR/13C NMR spectroscopy, IR spectroscopy, and
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service