C95803
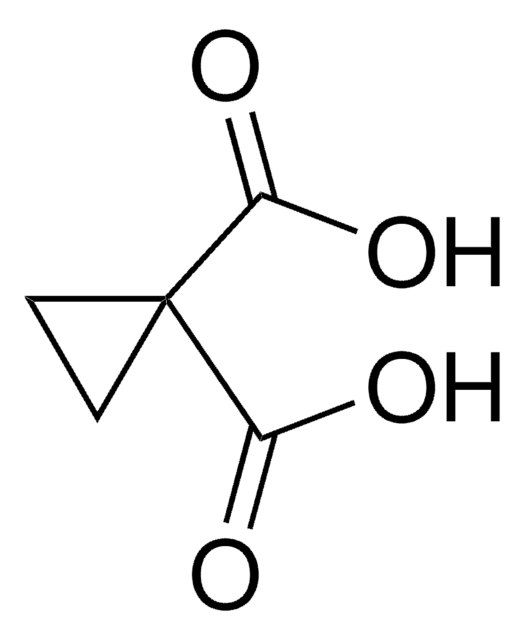
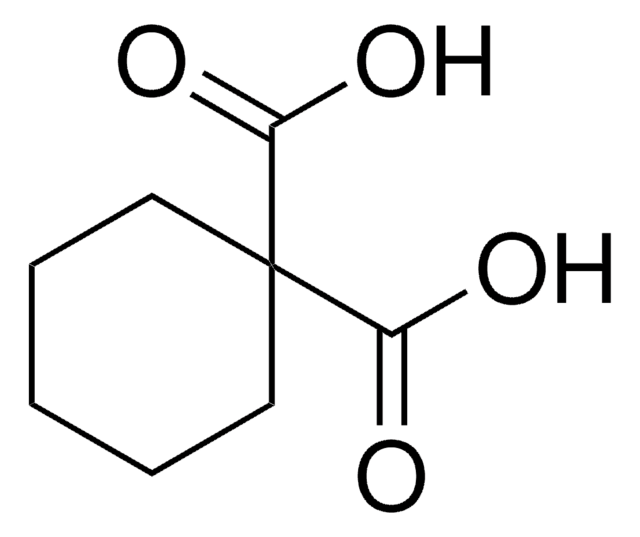
Cyclobutane-1,1-dicarboxylic acid
99%
Synonym(s):
1,1-Dicarboxycyclobutane, 1-Carboxycyclobutanecarboxylic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C6H8O4
CAS Number:
Molecular Weight:
144.13
Beilstein/REAXYS Number:
2046031
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
99%
form
crystals
mp
158 °C (lit.)
SMILES string
OC(=O)C1(CCC1)C(O)=O
InChI
1S/C6H8O4/c7-4(8)6(5(9)10)2-1-3-6/h1-3H2,(H,7,8)(H,9,10)
InChI key
CCQPAEQGAVNNIA-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Cyclobutane-1,1-dicarboxylic acid can be used as:
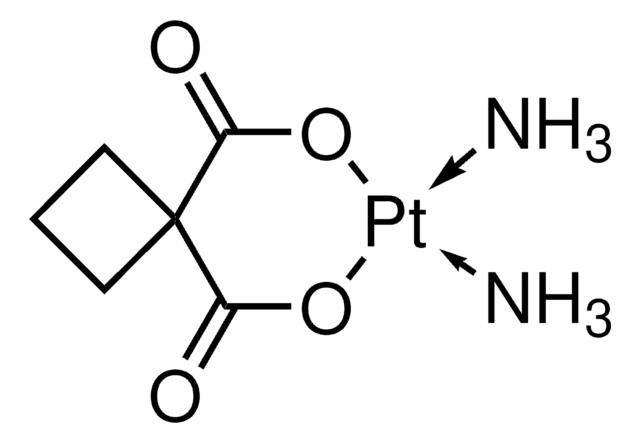
It can also be used as a starting material in the synthesis of carboplatin (cis-diammine(1,1-cyclobutanedicarboxyl-ate)platinum(II)).
- A reactant to synthesize lithium coordination polymer by treating with lithium carbonate.
- A ligand to prepare various lanthanide metal-organic frameworks (LnMOFs) via a solvothermal method. Eu+3 based MOF is applicable as a selective chemical sensor for detecting CH3OH due to its high luminescence quantum yield.
It can also be used as a starting material in the synthesis of carboplatin (cis-diammine(1,1-cyclobutanedicarboxyl-ate)platinum(II)).
signalword
Danger
hcodes
Hazard Classifications
Skin Corr. 1B
Storage Class
8A - Combustible corrosive hazardous materials
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Ivan V Kurganskii et al.
Molecules (Basel, Switzerland), 24(24) (2019-12-19)
Vanadium(IV) complexes are actively studied as potential candidates for molecular spin qubits operating at room temperatures. They have longer electron spin decoherence times than many other transition ions, being the key property for applications in quantum information processing. In most
Eman Mohamed Shoukry
Bioinorganic chemistry and applications, 512938-512938 (2009-08-22)
The [Pd(DEEN)Cl(2)] and [Pt(DEEN)Cl(2)] complexes were synthesized and characterized where DEEN = N,N-diethylethylenediamine. The stoichiometry and stability of the complexes formed between various biologically relevant ligands (amino acids, peptides, DNA constituents and dicarboxylic acids) and [Pd(DEEN)(H(2)O)(2)](2+) were investigated at 37
Soumava Biswas et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 21(39), 13793-13801 (2015-08-15)
Three isostructural lanthanide-based two- dimensional coordination polymers (CPs) {[Ln2(L)3(H2O)2]n⋅2n CH3OH)⋅2n H2O} (Ln=Gd(3+) (1), Tb(3+) (2), Dy(3+) (3); H2L=cyclobutane-1,1-dicarboxylic acid) were synthesized by using a low molecular weight dicarboxylate ligand and characterized. Single-crystal structure analysis showed that in complexes 1-3 lanthanide centers are
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service