D204307
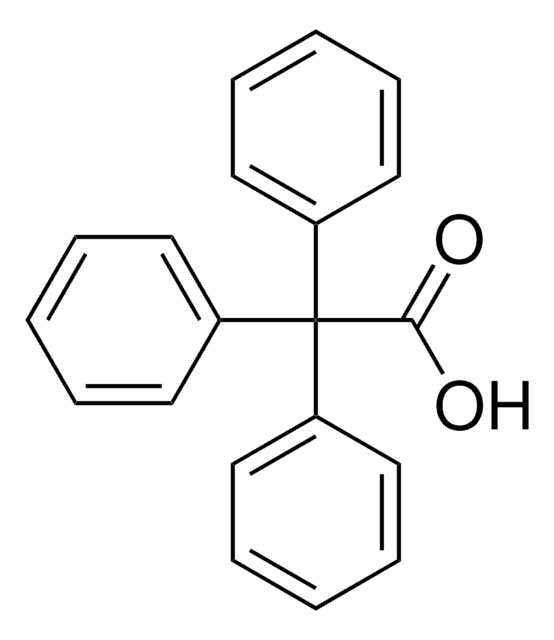
Diphenylacetic acid
99%
Synonym(s):
α,α-Diphenylacetic acid, α-Phenylbenzeneacetic acid, 2,2-Diphenylacetic acid, Diphenylethanoic acid
About This Item
Recommended Products
Quality Level
assay
99%
form
powder
mp
146-149 °C
147-149 °C (lit.)
SMILES string
OC(=O)C(c1ccccc1)c2ccccc2
InChI
1S/C14H12O2/c15-14(16)13(11-7-3-1-4-8-11)12-9-5-2-6-10-12/h1-10,13H,(H,15,16)
InChI key
PYHXGXCGESYPCW-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
It can also be used as:
- An additive in the ortho-arylation of 1-phenyl-β-carbolines using various aryl halides in the presence of ruthenium catalyst.
- A catalyst to synthesize 2-allyl-3-oxazolin-5-one derivatives via Rh-catalyzed coupling reaction of azlactones and alkynes followed by aza-Cope rearrangement.
signalword
Warning
hcodes
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Chronic 3 - Eye Irrit. 2
Storage Class
11 - Combustible Solids
wgk_germany
WGK 2
flash_point_f
383.5 °F - closed cup
flash_point_c
195.3 °C - closed cup
ppe
Eyeshields, Gloves, type N95 (US)
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service