F1506
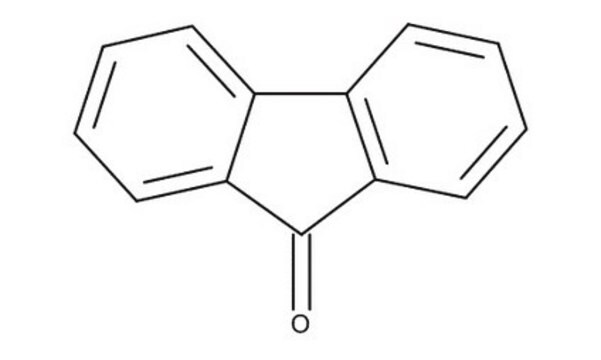
9-Fluorenone
98%
Synonym(s):
9H-Fluorene-9-one, Fluoren-9-one (8CI)
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C13H8O
CAS Number:
Molecular Weight:
180.20
Beilstein/REAXYS Number:
1636531
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
98%
bp
342 °C (lit.)
mp
80-83 °C (lit.)
SMILES string
O=C1c2ccccc2-c3ccccc13
InChI
1S/C13H8O/c14-13-11-7-3-1-5-9(11)10-6-2-4-8-12(10)13/h1-8H
InChI key
YLQWCDOCJODRMT-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
9-Fluorenone has been extensively used as a precursor to synthesize a variety of organic electronic materials. Some of the general examples are:
- Synthesis of host for the blue and green phosphorescent organic light emitting diodes (PHOLEDs).
- Synthesis of fluorene-based molecular motors.
- Synthesis of open-shell Chichibabin′s hydrocarbons as potential organic spintronic materials.
- It also acts as a sensitizer in the formation of picene via photosensitization of 1,2-di(1-naphthyl)ethane.
signalword
Warning
hcodes
Hazard Classifications
Aquatic Chronic 2 - Eye Irrit. 2
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
325.4 °F
flash_point_c
163 °C
ppe
Eyeshields, Gloves, type N95 (US)
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Jian Zhao et al.
Journal of the American Chemical Society, 129(16), 5288-5295 (2007-04-03)
Biologically interesting fluoren-9-one and xanthen-9-one derivatives have been prepared by a novel aryl to imidoyl palladium migration, followed by intramolecular arylation. The fluoren-9-one synthesis appears to involve both a palladium migration mechanism and a C-H activation process proceeding through an
Harmonizing triplet level and ambipolar characteristics of wide-gap phosphine oxide hosts toward highly efficient and low driving voltage blue and green PHOLEDs: an effective strategy based on spiro-systems.
Zhao J, et al.
Chemistry of Materials, 23(24), 5331-5339 (2011)
Tuning the rotation rate of light-driven molecular motors.
Bauer J, et al.
The Journal of Organic Chemistry, 79(10), 4446-4455 (2014)
T Atsumi et al.
Journal of oral rehabilitation, 31(12), 1155-1164 (2004-11-17)
Camphorquinone (CQ) is widely used as a photo-initiator in dental materials; however, its cytotoxicity against human pulp fibroblasts (HPF) and particularly the effects of 2-dimethylaminoethyl methacrylate (DMA), a reducing agent and visible light (VL) irradiation on it remain unknown. So
Dustin Pagoria et al.
Biomaterials, 26(19), 4091-4099 (2005-01-25)
Recent evidence suggests that following visible-light (VL) irradiation, CQ and the CQ-related photosensitizers benzil (BZ), benzophenone (BP), and 9-fluorenone (9-F) generate initiating radicals that may indiscriminately react with molecular oxygen forming reactive oxygen species (ROS). The purpose of this investigation
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service