H24003
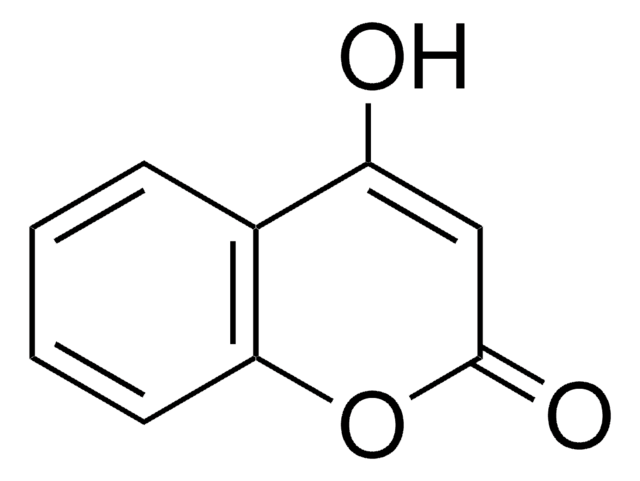
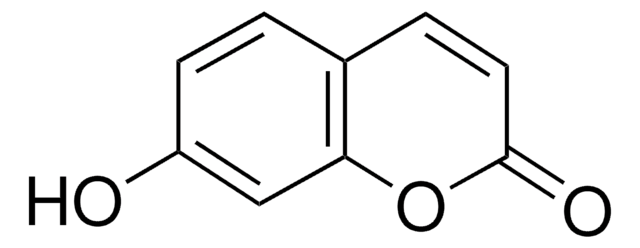
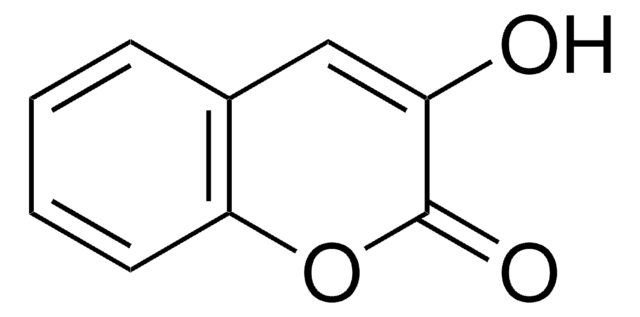
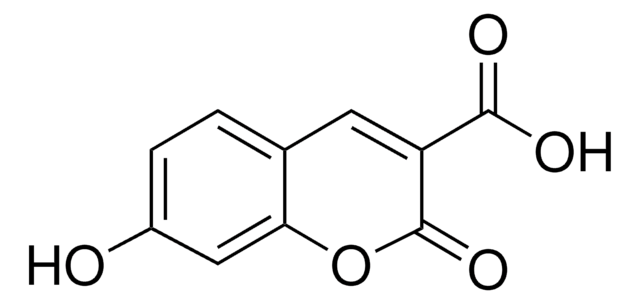
Umbelliferone
99%
Synonym(s):
7-Hydroxy-2H-1-benzopyran-2-one, 7-Hydroxycoumarin
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C9H6O3
CAS Number:
Molecular Weight:
162.14
Beilstein/REAXYS Number:
127683
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
99%
mp
230 °C (dec.) (lit.)
solubility
dioxane: soluble
ethanol: soluble
SMILES string
Oc1ccc2C=CC(=O)Oc2c1
InChI
1S/C9H6O3/c10-7-3-1-6-2-4-9(11)12-8(6)5-7/h1-5,10H
InChI key
ORHBXUUXSCNDEV-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Umbelliferone can be used:
- To synthesize fluorescent probes to detect cyanide anions in water at biological pH and for in vitro and in vivo detection of hydrogen peroxide.
- In the preparation of coumarin-based polymer as optical data storage material.
- As a precursor to synthesize novobiocin derivatives that exhibit antibacterial agents as well as inhibitors of DNA gyrase.
- As the starting material to synthesize (+)-decursinol and (+)-angelmarin.
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
ppe
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Fluorescent chemodosimeter for selective detection of cyanide in water.
Lee K S, et al.
Organic Letters, 10(1), 49-51 (2008)
Reversible two-photon optical data storage in coumarin-based copolymers.
Iliopoulos K, et al.
Journal of the American Chemical Society, 132(41), 14343-14345 (2010)
An efficient synthesis of (+)-decursinol from umbelliferone.
Lee J H, et al.
Tetrahedron Letters, 48(16), 2889-2892 (2007)
Rational design of a fluorescent hydrogen peroxide probe based on the umbelliferone fluorophore.
Du L, et al.
Tetrahedron Letters, 49(19), 3045-3048 (2008)
Novobiocin: redesigning a DNA gyrase inhibitor for selective inhibition of hsp90.
Burlison J A, et al.
Journal of the American Chemical Society, 128(48), 15529-15536 (2006)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service