H8706
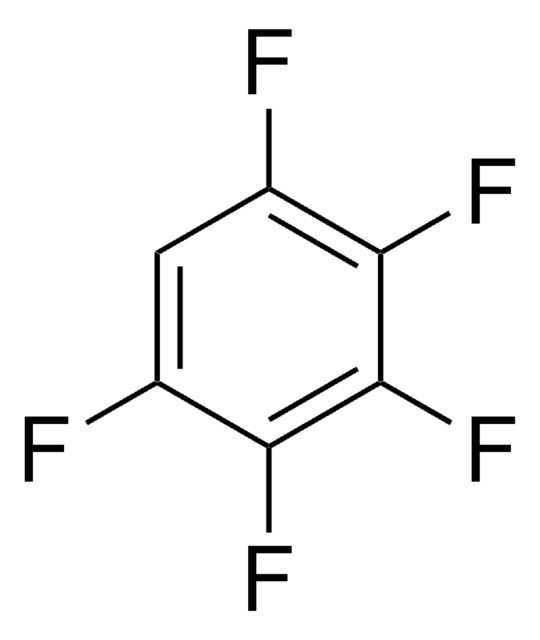
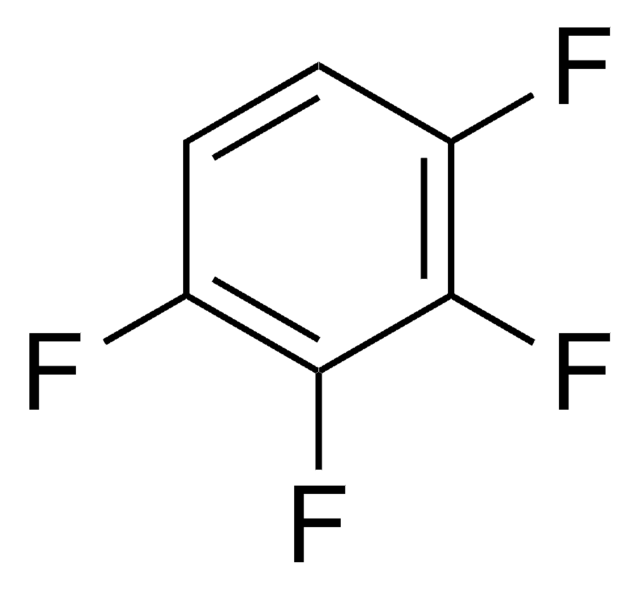
Hexafluorobenzene
99%
Synonym(s):
Perfluorobenzene
Sign Into View Organizational & Contract Pricing
Select a Size
All Photos(2)
Select a Size
Change View
About This Item
Empirical Formula (Hill Notation):
C6F6
CAS Number:
Molecular Weight:
186.05
Beilstein/REAXYS Number:
1683438
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
99%
form
liquid
refractive index
n20/D 1.377 (lit.)
bp
80-82 °C (lit.)
mp
3.7-4.1 °C (lit.)
density
1.612 g/mL at 25 °C (lit.)
SMILES string
Fc1c(F)c(F)c(F)c(F)c1F
InChI
1S/C6F6/c7-1-2(8)4(10)6(12)5(11)3(1)9
InChI key
ZQBFAOFFOQMSGJ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Hexafluorobenzene can react with:
It can be used:
- Ethyl magnesium bromide in the presence of transition metal halides to form the corresponding perfluoroarylmagnesium compound that can undergo Grignard reactions.
- The sodium salt of the appropriate phenol in 1,3-dimethyl-2-imidazolidinone (DMEU) to form the corresponding hexakis(aryloxy)benzenes.
It can be used:
- As a ligand to synthesize novel ruthenium(0) and osmium(0) hexafluorobenzene complexes.
- As a solvent and promoter for the ring-closing metathesis (RCM) to form tetrasubstituted olefins in the presence of a ruthenium-based catalyst.
signalword
Danger
hcodes
Hazard Classifications
Flam. Liq. 2
Storage Class
3 - Flammable liquids
wgk_germany
WGK 3
flash_point_f
50.0 °F - closed cup
flash_point_c
10 °C - closed cup
ppe
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
A hexafluorobenzene promoted ring-closing metathesis to form tetrasubstituted olefins.
Rost D, et al.
Tetrahedron Letters, 49(41), 5968-5971 (2008)
Synthesis of new ? 4-hexafluorobenzene complexes of ruthenium and osmium from atoms of the metals: crystal structure of [Ru (? 6-C 6 H 3 Me 3-1, 3, 5)(? 4-C 6 F 6)]
Martin A, et al.
Journal of the Chemical Society, (15), 2251-2255 (1994)
Synthesis of hexakis (aryloxy) benzenes: x-ray analysis of hexakis (phenyloxy) benzene and of the acetonitrile clathrate of hexakis (3, 5-dimethylphenyloxy) benzene
Gilmore C J, et al.
Tetrahedron Letters, 24(31), 3269-3272 (1983)
A new synthesis of perfluoroaromatic Grignard reagents.
Respess W L, et al.
Journal of Organometallic Chemistry, 18(2), 263-274 (1969)
Markus Allesch et al.
The journal of physical chemistry. B, 111(5), 1081-1089 (2007-02-03)
We report on the aqueous hydration of benzene and hexafluorobenzene, as obtained by carrying out extensive (>100 ps) first principles molecular dynamics simulations. Our results show that benzene and hexafluorobenzene do not behave as ordinary hydrophobic solutes, but rather present
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service