I5109
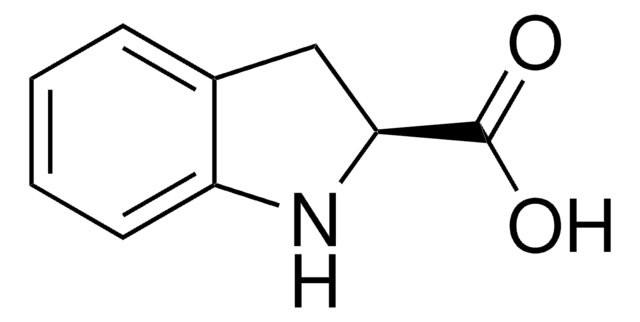
Indole-2-carboxylic acid
98%
Synonym(s):
2-Carboxyindole, 2-Indolylformic acid, NSC 16598
Sign Into View Organizational & Contract Pricing
All Photos(4)
About This Item
Empirical Formula (Hill Notation):
C9H7NO2
CAS Number:
Molecular Weight:
161.16
Beilstein/REAXYS Number:
124132
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
98%
mp
202-206 °C (lit.)
SMILES string
OC(=O)c1cc2ccccc2[nH]1
InChI
1S/C9H7NO2/c11-9(12)8-5-6-3-1-2-4-7(6)10-8/h1-5,10H,(H,11,12)
InChI key
HCUARRIEZVDMPT-UHFFFAOYSA-N
Gene Information
human ... SRD5A1(6715)
rat ... Grin2a(24409)
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
- Reactant for total synthesis of (±)-dibromophakellin and analogs
- Reactant for synthesis of the pyrrolizidine alkaloid (±)-trachelanthamidine
- Reactant for stereoselective preparation of renieramycin G analogs
- Reactant for preparation of spirooxoindolepyrrolidines via reduction of indole-2-carboxylic acid followed by oxidation, condensation, reduction, amidation and Kharasch radical cyclization
- Reactant for Pd-catalyzed cyclization
- Reactant for preparation of N,N′-(pentane)diylbis[indolecarboxamide] and N,N′-[phenylenebis(methylene)]bis[indolecarboxamide] derivatives
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Stephen P Nighswander-Rempel et al.
Photochemistry and photobiology, 84(3), 613-619 (2008-01-23)
We have synthesized a compound ideally suited to the study of structure-function relationships in eumelanin synthesis. N-methyl-5-hydroxy-6-methoxy-indole (MHMI) has key functional groups strategically placed on the indole framework to hinder binding in the 2, 5, 6 and 7 positions. Thus
C Kuehm-Caubere et al.
Journal of medicinal chemistry, 40(8), 1201-1210 (1997-04-11)
Series of indole-2-carboxamide and cycloalkeno[1,2-b]indole derivatives were synthesized and evaluated in order to determine the necessary structural requirements for a high inhibition of human LDL copper-induced peroxidation. Various modulations were systematically performed on the indole and cycloalkeno[1,2-b]indole nuclei as well
R Di Fabio et al.
Journal of medicinal chemistry, 40(6), 841-850 (1997-03-14)
A series of indole-2-carboxylates bearing suitable chains at the C-3 position of the indole nucleus was synthesized and evaluated in terms of in vitro affinity using [3H]glycine binding assay and in vivo potency by inhibition of convulsions induced by N-methyl-D-aspartate
Hideyuki Shiozawa et al.
Journal of the American Chemical Society, 124(15), 3914-3919 (2002-04-11)
Glycopeptide antibiotics of the vancomycin group bind to bacterial cell wall analogue precursors, and typically also form dimers. We have studied the interplay between these two sets of noncovalent bonds formed at separate interfaces. Indole-2-carboxylic acid (L) forms a set
R Rama Suresh et al.
The Journal of organic chemistry, 77(16), 6959-6969 (2012-07-26)
Two methodologies, one involving Ar-I reactivity and the other through C-H functionalization, for the formation of indolo[2,3-c]pyrane-1-ones via the corresponding allenes, are presented. A highly efficient approach to indolo[2,3-c]pyrane-1-one derivatives through the Pd-catalyzed regioselective annulation of allenes with 3-iodo-1-alkylindole-2-carboxylic acids
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service