N12805
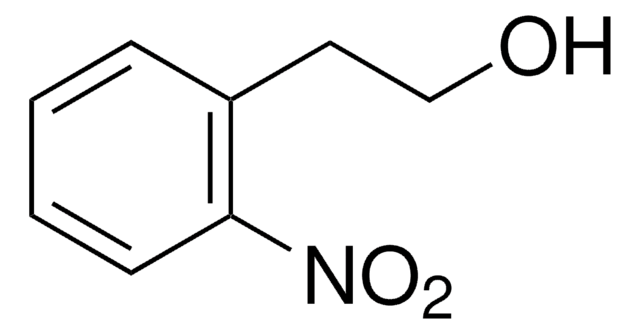
2-Nitrobenzyl alcohol
97%
Synonym(s):
(2-Nitrophenyl)methanol, 2-Nitrobenzenemethanol, o-(Hydroxymethyl)nitrobenzene, o-Nitrobenzyl alcohol
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
O2NC6H4CH2OH
CAS Number:
Molecular Weight:
153.14
Beilstein/REAXYS Number:
2046649
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
97%
form
solid
bp
270 °C (lit.)
mp
69-72 °C (lit.)
SMILES string
OCc1ccccc1[N+]([O-])=O
InChI
1S/C7H7NO3/c9-5-6-3-1-2-4-7(6)8(10)11/h1-4,9H,5H2
InChI key
BWRBVBFLFQKBPT-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Jie Cao et al.
Oncotarget, 7(50), 82170-82184 (2016-07-02)
A combination of chemo- and photo-thermal therapy (PTT) has provided a promising efficient approach for cancer therapy. To achieve the superior synergistic chemotherapeutic effect with PTT, the development of a simple theranostic nanoplatform that can provide both cancer imaging and
Record Broken: A Copper Peroxide Complex with Enhanced Stability and Faster Hydroxylation Catalysis.
Patricia Liebhäuser et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 23(50), 12171-12183 (2017-04-21)
Tyrosinase model systems pinpoint pathways to translating Nature's synthetic abilities for useful synthetic catalysts. Mostly, they use N-donor ligands which mimic the histidine residues coordinating the two copper centres. Copper complexes with bis(pyrazolyl)methanes with pyridinyl or imidazolyl moieties are already
Claudia Wilfer et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 21(49), 17639-17649 (2015-10-13)
Bis(pyrazolyl)methane ligands are excellent components of model complexes used to investigate the activity of the enzyme tyrosinase. Combining the N donors 3-tert-butylpyrazole and 1-methylimidazole results in a ligand that is capable of stabilising a (μ-η(2) :η(2) )-dicopper(II) core that resembles
Wanting Hou et al.
Molecules (Basel, Switzerland), 25(21) (2020-11-11)
Due to a strong retardation effect of o-nitrobenzyl ester on polymerization, it is still a great challenge to prepare amphiphilic block copolymers for polymersomes with a o-nitrobenzyl ester-based hydrophobic block. Herein, we present one such solution to prepare amphiphilic block
Martin Gaplovsky et al.
Photochemical & photobiological sciences : Official journal of the European Photochemistry Association and the European Society for Photobiology, 4(1), 33-42 (2004-12-24)
Irradiation of 2-nitrobenzyl alcohol (1, R = H) and 1-(2-nitrophenyl)ethanol (1, R = Me) in various solvents yields 2-nitroso benzaldehyde (4, R = H) and 2-nitroso acetophenone (4 R = Me), respectively, with quantum yields of about 60%. The mechanism
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service


![4-[4-(1-Hydroxyethyl)-2-methoxy-5-nitrophenoxy]butyric acid ≥98.0% (HPLC)](/deepweb/assets/sigmaaldrich/product/structures/232/152/e26ea38f-f1d4-4f88-a61b-466cd10aa1dc/640/e26ea38f-f1d4-4f88-a61b-466cd10aa1dc.png)