N7206
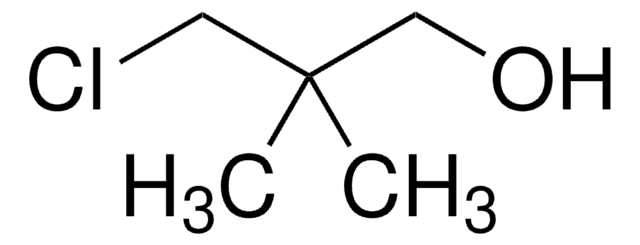
2,2-Dimethyl-1-propanol
99%
Synonym(s):
tert-Butylmethanol, Neoamyl alcohol, Neopentanol, tert-Butyl carbinol, Neopentyl alcohol
About This Item
Recommended Products
vapor pressure
16 mmHg ( 20 °C)
assay
99%
form
crystals
bp
113-114 °C (lit.)
mp
52-56 °C (lit.)
density
0.818 g/mL at 25 °C (lit.)
SMILES string
CC(C)(C)CO
InChI
1S/C5H12O/c1-5(2,3)4-6/h6H,4H2,1-3H3
InChI key
KPSSIOMAKSHJJG-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
- 2,2-Dimethyl-1-propanol can be used in the synthesis of surfactant that stabilize reduced graphene oxide (rGO) dispersion.
- Biodiesel containing branched-chain esters prepared by the transesterification of vegetable oils with 2,2-dimethyl-1-propanol has been reported to show lower crystallization temperature when compared to methyl and ethyl ester counterparts.
signalword
Danger
hcodes
Hazard Classifications
Acute Tox. 4 Inhalation - Flam. Sol. 1 - STOT SE 3
target_organs
Respiratory system
Storage Class
4.1B - Flammable solid hazardous materials
wgk_germany
WGK 1
flash_point_f
82.4 °F - closed cup
flash_point_c
28 °C - closed cup
ppe
Eyeshields, Gloves, type P3 (EN 143) respirator cartridges
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service