P45605
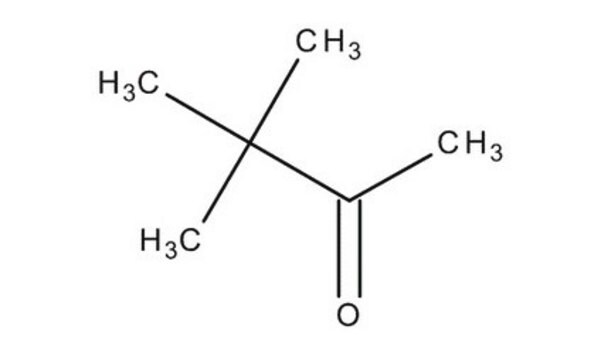
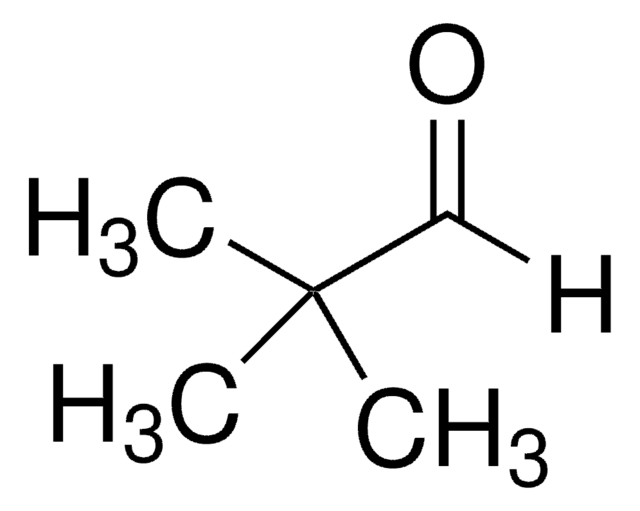
3,3-Dimethyl-2-butanone
97%
Synonym(s):
α,α,α-Trimethylacetone, tert-Butyl methyl ketone, Pinacolone
Sign Into View Organizational & Contract Pricing
Select a Size
All Photos(2)
Select a Size
Change View
About This Item
Linear Formula:
CH3COC(CH3)3
CAS Number:
Molecular Weight:
100.16
Beilstein/REAXYS Number:
1209331
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
97%
refractive index
n20/D 1.396 (lit.)
bp
106 °C (lit.)
density
0.801 g/mL at 25 °C (lit.)
SMILES string
CC(=O)C(C)(C)C
InChI
1S/C6H12O/c1-5(7)6(2,3)4/h1-4H3
InChI key
PJGSXYOJTGTZAV-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
3,3-Dimethyl-2-butanone is an aliphatic ketone can undergo asymmetric reduction to the corresponding alcohol with diisopinocampheylchloroborane with high enantiomeric excess.
signalword
Danger
hcodes
Hazard Classifications
Acute Tox. 4 Oral - Flam. Liq. 2
Storage Class
3 - Flammable liquids
wgk_germany
WGK 2
flash_point_f
41.0 °F - closed cup
flash_point_c
5 °C - closed cup
ppe
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Richard E Mishler et al.
Chemical communications (Cambridge, England), (41)(41), 6201-6203 (2009-10-15)
We report a new and simple one-pot synthetic method to produce mesoporous silica and nanoporous solid acid catalyst capable of catalyzing pinacole-pinacolone rearrangement and esterification reactions, by preparing a solvent washable phosphonated triblock copolymer template and self-assembling it in the
Makoto Shimizu et al.
Organic letters, 4(23), 4097-4099 (2002-11-09)
The pinacol reaction of beta-halogenated alpha,beta-unsaturated aldehydes was promoted by titanium tetraiodide to give coupling products in good yields with high dl-selectivity. Subsequent reduction with H(2)/Pd-C gave saturated vic-diols in good yields. Heck coupling reaction enabled the displacement of halogens
Direct conversion of arylamines to pinacol boronates: a metal-free borylation process.
Fanyang Mo et al.
Angewandte Chemie (International ed. in English), 49(10), 1846-1849 (2010-02-04)
Y Chen et al.
The Journal of organic chemistry, 66(11), 3930-3939 (2001-05-26)
To investigate the effects of electron-donating and electron-withdrawing substituents upon the reaction of porphyrins with osmium tetraoxide, and the pinacol-pinacolone rearrangement of the resulting diols, a series of meso-substituted porphyrins were prepared by total synthesis. Porphyrins with electron-donating substitutents at
Keliang Gao et al.
Applied microbiology and biotechnology, 71(6), 819-823 (2006-02-21)
Enantioselective biotransformation of DL-1,2-propanediol to D-2-hydroxypropanic acid was first reported by the authors. In the biooxidation process, there were some by-product formed and thus influenced the e.e. value and output of the acid. Restricting oxygen in the reaction system and
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service