S4921
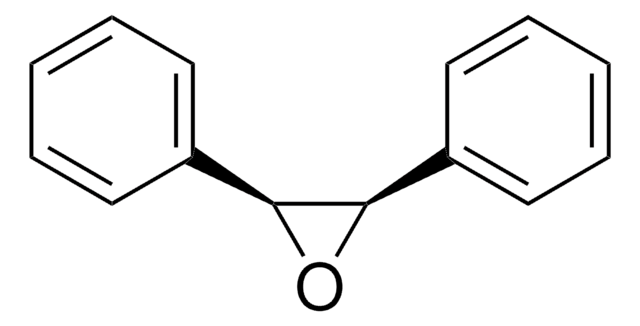
trans-Stilbene oxide
98%
Synonym(s):
trans-1,2-Diphenyloxirane
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C14H12O
CAS Number:
Molecular Weight:
196.24
Beilstein/REAXYS Number:
82740
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
98%
mp
65-67 °C (lit.)
SMILES string
O1[C@@H]([C@H]1c2ccccc2)c3ccccc3
InChI
1S/C14H12O/c1-3-7-11(8-4-1)13-14(15-13)12-9-5-2-6-10-12/h1-10,13-14H/t13-,14-/m1/s1
InChI key
ARCJQKUWGAZPFX-ZIAGYGMSSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Trans-stilbene oxide also known as trans-1,2-Diphenyloxirane, is often used in photochemistry, whereit can change its structure when exposed to light.Stilbene oxides can break apart when they are excited bylight, leading to the formation of carbonyl ylides. It is also commonlyused to produce trans-stilbene sulfides.
Application
- Chiral Stationary Phases for Liquid Chromatography: Trans-stilbene oxide has been utilized in the fabrication of cellulose derivative-coated spherical covalent organic frameworks, serving as chiral stationary phases for high-performance liquid chromatographic enantioseparation, demonstrating its pivotal role in advanced analytical methodologies (Yan et al., 2022).
- Method Selection for Chiral High-Performance Liquid Chromatography: Its application extends to the utilization of hysteresis phenomena for chiral high-performance liquid chromatographic method selection in polar organic mode, enhancing the efficiency and specificity of pharmaceutical compound analysis (Horváth et al., 2020).
- Adsorption Properties for Enantioseparations: The effect of chiral selector loading on the adsorption properties of fully- and superficially-porous particles is crucial for high-efficient ultrafast enantioseparations, where trans-stilbene oxide derivatives play a significant role (Felletti et al., 2018).
- Catalysis in Alkene Epoxidation: Trans-stilbene oxide is involved in innovative catalysis research, specifically in the development of carbon nitride-supported Fe(2) cluster catalysts for alkene epoxidation, showcasing its utility in sustainable chemical synthesis (Tian et al., 2018).
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Photochemistry of cis-and trans-stilbene oxides
Lee, George A
The Journal of Organic Chemistry, 41, 2656-2658 (1976)
S Bernardini et al.
Mutagenesis, 16(3), 277-281 (2001-04-26)
About 50% and 15% of Caucasians lack the glutathione S-transferase M1 (GSTM1) and T1 (GSTT1) genes and the corresponding enzyme activity, respectively. Both of these polymorphisms have been shown to affect the genotoxicity of some epoxides in cultured human lymphocytes.
Kouhei Shimomura et al.
Nature chemistry, 6(5), 429-434 (2014-04-24)
In the chromatographic separation of enantiomers the order of elution is determined by the strength of diasteromeric interactions between the components of the mixture and a chiral stationary phase. For analytical purposes, it is ideal to have the minor component
Richard Lonsdale et al.
Biochemistry, 51(8), 1774-1786 (2012-01-28)
Soluble epoxide hydrolase (sEH) is an enzyme involved in drug metabolism that catalyzes the hydrolysis of epoxides to form their corresponding diols. sEH has a broad substrate range and shows high regio- and enantioselectivity for nucleophilic ring opening by Asp333.
B Schilter et al.
The Journal of pharmacology and experimental therapeutics, 294(3), 916-922 (2000-08-17)
Oxidative biotransformation, coupled with genetic variability in enzyme expression, has been the focus of hypotheses interrelating environmental and genetic factors in the etiology of central nervous system disease processes. Chemical modulation of cerebral cytochrome P450 (P450) monooxygenase expression character may
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service