M-077
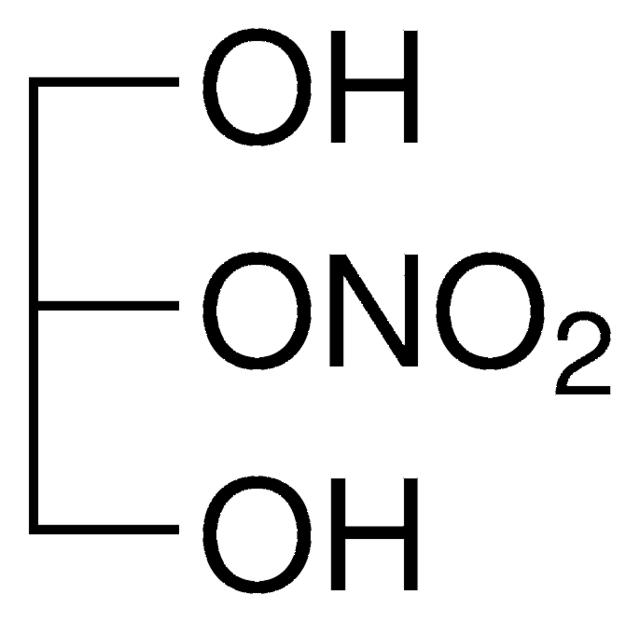
1-Mononitroglycerin solution
1.0 mg/mL in acetonitrile, ampule of 1 mL, certified reference material, Cerilliant®
About This Item
Recommended Products
grade
certified reference material
Quality Level
form
liquid
feature
Snap-N-Spike®/Snap-N-Shoot®
packaging
ampule of 1 mL
manufacturer/tradename
Cerilliant®
concentration
1.0 mg/mL in acetonitrile
technique(s)
gas chromatography (GC): suitable
liquid chromatography (LC): suitable
application(s)
pharmaceutical (small molecule)
format
single component solution
storage temp.
−20°C
SMILES string
O[C@H](CO)CO[N+]([O-])=O
InChI
1S/C3H7NO5/c5-1-3(6)2-9-4(7)8/h3,5-6H,1-2H2
InChI key
HXWLJBVVXXBZCM-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
- Growth of Arthrobacter sp. strain JBH1 on Nitroglycerin: This study highlights the novel capability of Arthrobacter sp. strain JBH1 to metabolize nitroglycerin as its sole source of carbon and nitrogen. The findings are critical for biotechnological applications aiming to biodegrade nitroglycerin residues in contaminated environments, presenting an effective bioremediation strategy for pollutants derived from pharmaceutical manufacturing processes (Husserl et al., 2010).
Legal Information
related product
signalword
Danger
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Irrit. 2 - Flam. Liq. 2
Storage Class
3 - Flammable liquids
wgk_germany
WGK 2
flash_point_f
35.6 °F - closed cup
flash_point_c
2 °C - closed cup
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service