8.51022
Dabcyl-OSu
Novabiochem®
Synonym(s):
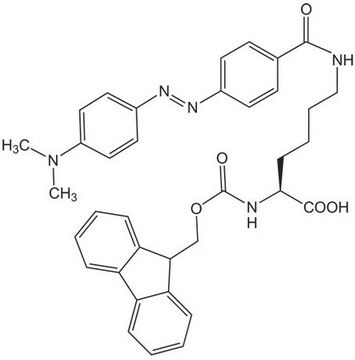
Dabcyl-OSu, N-(4-[4Æ-(Dimethylamino)phenylazo]benzoyloxy)succinimide
About This Item
Recommended Products
Quality Level
product line
Novabiochem®
assay
≥98.0% (HPLC)
form
powder
reaction suitability
reagent type: fluorescent-labelling reagent
manufacturer/tradename
Novabiochem®
application(s)
peptide synthesis
storage temp.
15-25°C
SMILES string
N3(C(=O)CCC3=O)OC(=O)c1ccc(cc1)N=Nc2ccc(cc2)N(C)C
InChI
1S/C19H18N4O4/c1-22(2)16-9-7-15(8-10-16)21-20-14-5-3-13(4-6-14)19(26)27-23-17(24)11-12-18(23)25/h3-10H,11-12H2,1-2H3
Related Categories
General description
Associated Protocols and Technical Articles
Chromogenic Labeling of Synthetic Peptides
Linkage
Analysis Note
Appearance of substance (visual): powder
Identity (IR): passes test
Purity (TLC(011A)): ≥ 98 %
Assay (HPLC, area%): ≥ 98.0 % (a/a)
To see the solvent systems used for TLC of Novabiochem® products please click here.
Legal Information
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
Chromogenic and fluorogenic derivatives are invaluable tools for biochemistry, having numerous applications in enzymology, protein chemistry, immunology and histochemistry.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service
![4-[4-(Dimethylamino)phenylazo]benzoic acid N-succinimidyl ester ≥98.0% (HPLC)](/deepweb/assets/sigmaaldrich/product/structures/120/235/500b5276-3ce2-43b7-9588-3883f13d4ff7/640/500b5276-3ce2-43b7-9588-3883f13d4ff7.png)