CH2900019
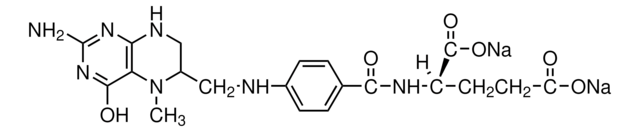
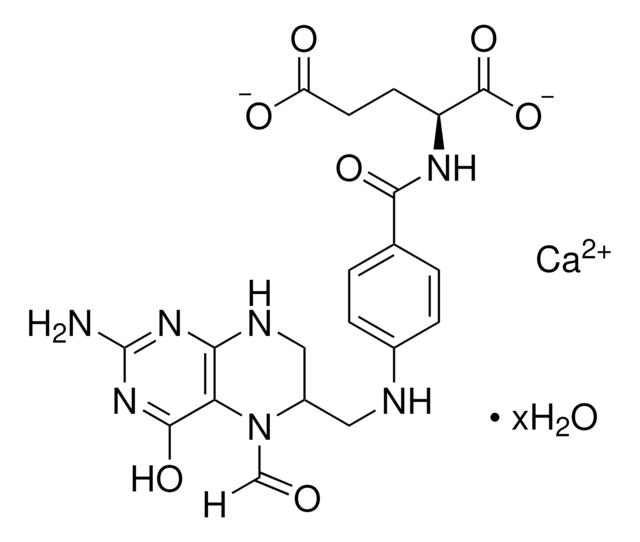
Metafolin® ( L-5-methyltetrahydrofolic acid calcium (L-5-MTHF-Ca))
L-5-methyltetrahydrofolic acid calcium (L-5-MTHF-Ca), USP
Pharma Manufacturing
Synonym(s):
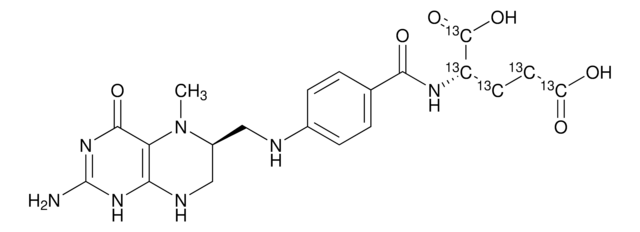
(6S)-5-Methyl-5,6,7,8-tetrahydropteroyl-L-glutamic acid, (6S)-5-Methyltetrahydrofolic acid, Calcium salt, Calcium salt [(6S)-5-MTHF-Ca], L-5-MTHF; L-5-MTHF-Ca, L-5-methyltetrahydrofolate, L-5-methyltetrahydrofolate-Calcium, L-5-methyltetrahydrofolic acid (L-5-MTHF-Ca)
About This Item
Recommended Products
biological source
synthetic
Quality Level
grade
GMO free
GMP
Halal
Kosher
agency
USP
form
powder
application(s)
pharmaceutical
solid formulation
vitamins, nutraceuticals, and natural products
storage temp.
2-8°C
General description
Application
Packaging
Legal Information
Application
also commonly purchased with this product
related product
Storage Class
11 - Combustible Solids
wgk_germany
WGK 1
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
Arcofolin® Methylfolate as a superior alternative to Folic Acid has uniquely become available for nutritional supplementation and fortification.
Medicine for children poses unique formulation challenges compared to adults. Consider developmental physiology and age specifics when designing pharmaceuticals. Quality issues can severely impact patient safety. Therefore, excipient quality, supplier selection, and supply chain security are crucial, particularly for pediatric formulations.
Related Content
Our high-quality folates are manufactured under cGMP guideline ICH Q7 and backed by dedicated regulatory support worldwide.
Developing formulations specifically for infants and children is increasingly important. To help you master these challenges effectively, we offer an extensive portfolio of well-established high-quality excipients and APIs for pediatric pharmaceutical formulations that are proven in practice.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service