MABN1839
Anti-Amyloid-β (oligomer) Antibody, clone F11G3
clone F11G3, from mouse
Synonym(s):
Amyloid beta oligomer, Abeta oligomer, alpha-synuclein oligomer,, alpha-syn oligomer, Prion protein oligomer, PrP oligomer, TAR DNA-binding protein 43 oligomer, TDP-43 oligomer, Tau oligomer
About This Item
Recommended Products
biological source
mouse
Quality Level
antibody form
purified immunoglobulin
antibody product type
primary antibodies
clone
F11G3, monoclonal
species reactivity
all, human, mouse
technique(s)
ELISA: suitable
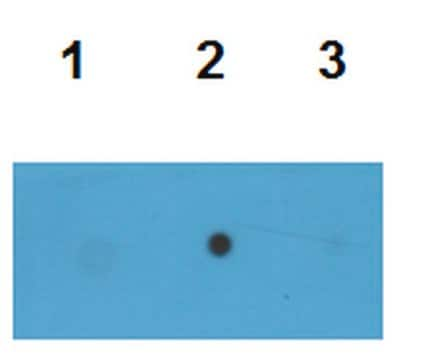
dot blot: suitable
immunofluorescence: suitable
immunoprecipitation (IP): suitable
western blot: suitable
isotype
IgMκ
NCBI accession no.
UniProt accession no.
General description
Specificity
Immunogen
Application
Western Blotting Analysis: A representative lot detected Ataxin-1 oligomers in soluble cerebella extracts from Atxn1154Q/+, but not wild-type or Atxn-/-, mice (Lasagna-Reeves, C.A., et al. (2015). eLlife. 4:e07558).
Western Blotting Analysis: A representative lot detected cellular beta-sheet oligomer immunoreactivity in HeLa cells transfected with the pathogenic (82Q), but not the non-pathogenic (30Q) form of polyQ Ataxin-1 in transfected Hela cells. Co-transfecting with the native Atxn-1 binding partner Capicua (CIC), but not the binding defective CIC W37A mutant, enhanced the oligomer formation (Lasagna-Reeves, C.A., et al. (2015). eLlife. 4:e07558).
Western Blotting Analysis: A representative lot detected the highest extend of oligomers accumulation in the soluble cerebella extracts among the 28-week old Atxn1154Q/+ mice when compared with samples from 18-week old and 8-week old Atxn1154Q/+ mice, with the 8-week old mice bearing the least oligomer buildup (Lasagna-Reeves, C.A., et al. (2015). eLlife. 4:e07558).
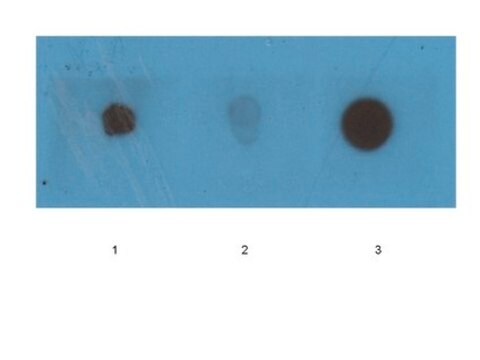
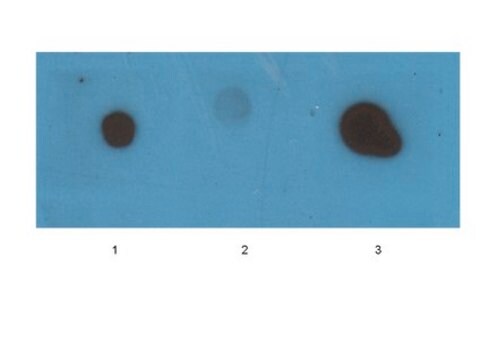
Western Blotting Analysis: A representative lot specifically detected oligomeric, but not monomeric or fibrillar, forms of Aβ42, α-Syn, PrP, and TDP-43 (Guerrero-Muñoz, M.J., et al. (2014). Neurobiol. Dis.71:14-23).
App3/DB/ A representative lot specifically detected oligomeric, but not monomeric or fibrillar, forms of Aβ42, α-Syn, PrP, and TDP-43 (Guerrero-Muñoz, M.J., et al. (2014). Neurobiol. Dis.71:14-23).
ELISA Analysis: A representative lot detected in vitro Aβ42, α-Syn, PrP, and TDP-43 oligomers formation with or without Aβ42 oligomer seeding (Guerrero-Muñoz, M.J., et al. (2014). Neurobiol. Dis.71:14-23).
Immunofluorescence Analysis: A representative lot detected a positive correlation between the ATXN1 beta-sheet oligomer immunoreactivity and the degeneration progression in calbindin-positive Purkinje cells (PCs) by fluorescent immunohistochemistry using paraffin-embedded cerebellum sections from Atxn1154Q/+ mice (Lasagna-Reeves, C.A., et al. (2015). eLlife. 4:e07558).
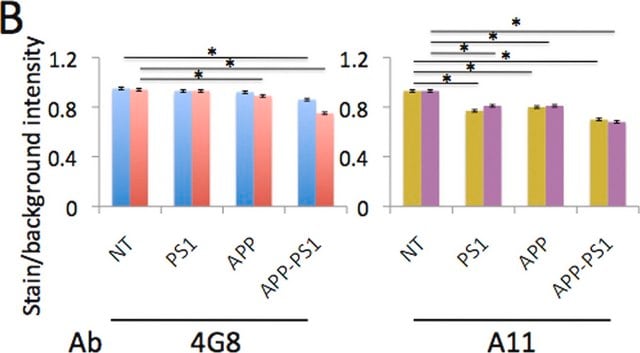
Immunofluorescence Analysis: A representative lot selectively detected beta-sheet oligomer immunoreactivity colocalized with those of Aβ, α-Syn, PrP, and TDP-43 in paraffin-embedded frontal cortex sections from Alzheimer′s diseased brain by fluorescent immunohistochemistry. The beta-sheet oligomer immunoreactivity is not detected in non-AD brains and is distinct from the staining pattern obtained with Thioflavin S (Guerrero-Muñoz, M.J., et al. (2014). Neurobiol. Dis.71:14-23).
Immunoprecipitation Analysis: A representative lot immunoprecipitated Ataxin-1 oligomers from the soluble cerebella extracts of Atxn1154Q/+, but not Atxn-/-, mice (Lasagna-Reeves, C.A., et al. (2015). eLlife. 4:e07558).
Immunocytochemistry Analysis: A representative lot detected cellular beta-sheet oligomer immunoreactivity in HeLa cells transfected with the pathogenic polyQ Ataxin-1 mRFP fusion construct mRFP-ATXN1(82Q) by fluorescent immunocytochemistry. Co-transfecting with the N-terminal fragment of the Atxn-1 binding partner Capicua (CIC), but not the binding defective CIC W37A mutant fragment, enhanced the oligomer formation (Lasagna-Reeves, C.A., et al. (2015). eLlife. 4:e07558).
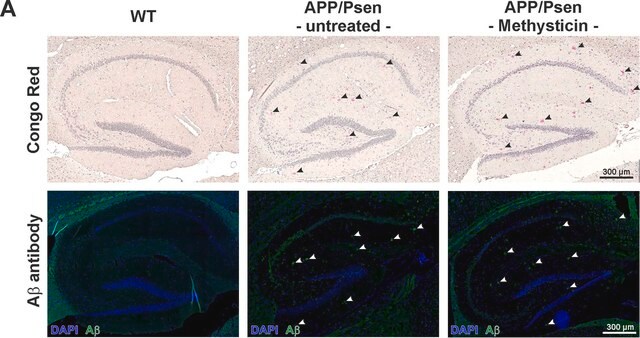
Immunohistochemistry Analysis: A representative lot detected beta-sheet oligomer immunoreactivity in paraffin-embedded cerebellum and cortex sections of Atxn1154Q/+, but not wild-type, mice (Lasagna-Reeves, C.A., et al. (2015). eLlife. 4:e07558).
Neuroscience
Neurodegenerative Diseases
Quality
Western Blotting Analysis: 2.0 µg/mL of this antibody detected 10 µg of oligomeric amyloid.
Target description
Physical form
Storage and Stability
Handling Recommendations: Upon receipt and prior to removing the cap, centrifuge the vial and gently mix the solution. Aliquot into microcentrifuge tubes and store at -20°C. Avoid repeated freeze/thaw cycles, which may damage IgG and affect product performance.
Other Notes
Disclaimer
Not finding the right product?
Try our Product Selector Tool.
recommended
Storage Class
12 - Non Combustible Liquids
wgk_germany
WGK 2
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service